Thyroid autoimmunity and ophthalmopathy related to melanoma biological therapy
- PMID: 21088057
- PMCID: PMC4080629
- DOI: 10.1530/EJE-10-0833
Thyroid autoimmunity and ophthalmopathy related to melanoma biological therapy
Abstract
Objective: Ipilimumab is a fully human MAB against cytotoxic T-lymphocyte antigen 4 (CTLA4). CTLA4 negatively regulates immune cell activation. In patients with metastatic melanoma, ipilimumab increases survival time and induces complete remission in some patients. However, immune-related adverse events including endocrinopathies have been reported. Bevacizumab, an angiogenesis inhibitor, has been used in combination with ipilimumab in patients with advanced melanoma.
Patients and methods: In this study, we report three patients who received ipilimumab alone or combined with bevacizumab therapy and developed thyroiditis, and the first report of euthyroid Graves' ophthalmopathy.
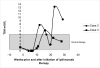
Results: Case 1 is a 51-year-old female who presented with severe eye pain, proptosis, and periorbital edema. Laboratory results revealed normal TSH, elevated thyroid antibodies but low titer of anti-TSH receptor antibody. Imaging was consistent with Graves' ophthalmopathy. Cases 2 and 3 were referred for hyperthyroidism, and workup revealed thyroiditis. These three cases suggest that patients with advanced melanoma treated with ipilimumab +/- bevacizumab may be susceptible to a variety of thyroid disorders.
Conclusions: Anti-CTLA4 therapy has shown promising results in treating advanced malignancy such as melanoma and renal carcinoma. A number of endocrinopathies, including thyroid disorders, may develop during ipilimumab therapy. The association of bevacizumab with endocrinopathies is not clear, although a few reports suggest a link to hypothyroidism. All patients on ipilimumab and/or bevacizumab therapy should be monitored for signs or symptoms of thyroiditis.
Figures
Similar articles
-
Thyroid-like ophthalmopathy in a euthyroid patient receiving Ipilimumab.Orbit. 2014 Dec;33(6):424-7. doi: 10.3109/01676830.2014.949792. Epub 2014 Sep 10. Orbit. 2014. PMID: 25207976
-
[Rare differential diagnosis of hyperthyroidism].Dtsch Med Wochenschr. 2016 Jun;141(12):889. doi: 10.1055/s-0041-104686. Epub 2016 Jun 15. Dtsch Med Wochenschr. 2016. PMID: 27305306 German.
-
Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution.Endocr Relat Cancer. 2014 Mar 7;21(2):371-81. doi: 10.1530/ERC-13-0499. Print 2014 Apr. Endocr Relat Cancer. 2014. PMID: 24610577 Free PMC article. Review.
-
Antibody-dependent cell-mediated cytotoxicity against orbital target cells in thyroid-associated ophthalmopathy and related disorders; close relationship between serum cytotoxic antibodies and parameters of eye muscle dysfunction.J Endocrinol Invest. 1996 Jun;19(6):334-41. doi: 10.1007/BF03344966. J Endocrinol Invest. 1996. PMID: 8844451
-
Ipilimumab: a novel immunomodulating therapy causing autoimmune hypophysitis: a case report and review.Eur J Endocrinol. 2012 Jul;167(1):1-5. doi: 10.1530/EJE-12-0167. Epub 2012 Apr 10. Eur J Endocrinol. 2012. PMID: 22495490 Review.
Cited by
-
The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network.PLoS One. 2013;8(1):e53745. doi: 10.1371/journal.pone.0053745. Epub 2013 Jan 14. PLoS One. 2013. PMID: 23341990 Free PMC article.
-
Ipilimumab: its potential in non-small cell lung cancer.Ther Adv Med Oncol. 2012 Mar;4(2):43-50. doi: 10.1177/1758834011431718. Ther Adv Med Oncol. 2012. PMID: 22423263 Free PMC article.
-
The Current Understanding of the Endocrine Effects From Immune Checkpoint Inhibitors and Recommendations for Management.JNCI Cancer Spectr. 2018 Jul;2(3):pky021. doi: 10.1093/jncics/pky021. Epub 2018 Jul 19. JNCI Cancer Spectr. 2018. PMID: 30057972 Free PMC article. Review.
-
Neuro-ophthalmic Complications of Immune Checkpoint Inhibitors: A Systematic Review.Eye Brain. 2020 Nov 3;12:139-167. doi: 10.2147/EB.S277760. eCollection 2020. Eye Brain. 2020. PMID: 33173368 Free PMC article. Review.
-
Graves' Disease during Immune Checkpoint Inhibitor Therapy (A Case Series and Literature Review).Cancers (Basel). 2021 Apr 17;13(8):1944. doi: 10.3390/cancers13081944. Cancers (Basel). 2021. PMID: 33920721 Free PMC article.
References
-
- Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol. 2001;19:565–594. - PubMed
-
- Blansfield JA, Beck KE, Tran K, Yang JC, Hughes MS, Kammula US, Royal RE, Topalian SL, Haworth LR, Levy C, Rosenberg SA, Sherry RM. Cytotoxic T-lymphocyte-associated antigen-4 blockage can induce autoimmune hypophysitis in patients with metastatic melanoma and renal cancer. J Immunother. 2005;28:593–598. - PMC - PubMed
-
- Dillard T, Yedinak CG, Alumkal J, Fleseriu M. Anti-CTLA-4 antibody therapy associated autoimmune hypophysitis: serious immune related adverse events across a spectrum of cancer subtypes. Pituitary. 2010;13:29–38. - PubMed
-
- Boehm S, Rothermundt C, Hess D, Joerger M. Antiangiogenic drugs in oncology: a focus on drug safety and the elderly - a mini-review. Gerontology. 2010;56:303–309. - PubMed
-
- Karantanis D, Bogsrud TV, Wiseman GA, Mullan BP, Subramaniam RM, Nathan MA, Peller PJ, Bahn RS, Lowe VJ. Clinical significance of diffusely increased 18F-FDG uptake in the thyroid gland. J Nucl Med. 2007;48:896–901. - PubMed