Herbivore-induced and floral homoterpene volatiles are biosynthesized by a single P450 enzyme (CYP82G1) in Arabidopsis
- PMID: 21088219
- PMCID: PMC3000306
- DOI: 10.1073/pnas.1009975107
Herbivore-induced and floral homoterpene volatiles are biosynthesized by a single P450 enzyme (CYP82G1) in Arabidopsis
Abstract
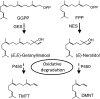
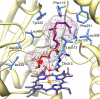
Terpene volatiles play important roles in plant-organism interactions as attractants of pollinators or as defense compounds against herbivores. Among the most common plant volatiles are homoterpenes, which are often emitted from night-scented flowers and from aerial tissues upon herbivore attack. Homoterpene volatiles released from herbivore-damaged tissue are thought to contribute to indirect plant defense by attracting natural enemies of pests. Moreover, homoterpenes have been demonstrated to induce defensive responses in plant-plant interaction. Although early steps in the biosynthesis of homoterpenes have been elucidated, the identity of the enzyme responsible for the direct formation of these volatiles has remained unknown. Here, we demonstrate that CYP82G1 (At3g25180), a cytochrome P450 monooxygenase of the Arabidopsis CYP82 family, is responsible for the breakdown of the C(20)-precursor (E,E)-geranyllinalool to the insect-induced C(16)-homoterpene (E,E)-4,8,12-trimethyltrideca-1,3,7,11-tetraene (TMTT). Recombinant CYP82G1 shows narrow substrate specificity for (E,E)-geranyllinalool and its C(15)-analog (E)-nerolidol, which is converted to the respective C(11)-homoterpene (E)-4,8-dimethyl-1,3,7-nonatriene (DMNT). Homology-based modeling and substrate docking support an oxidative bond cleavage of the alcohol substrate via syn-elimination of the polar head, together with an allylic C-5 hydrogen atom. CYP82G1 is constitutively expressed in Arabidopsis stems and inflorescences and shows highly coordinated herbivore-induced expression with geranyllinalool synthase in leaves depending on the F-box protein COI-1. CYP82G1 represents a unique characterized enzyme in the plant CYP82 family with a function as a DMNT/TMTT homoterpene synthase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
The biochemistry of homoterpenes--common constituents of floral and herbivore-induced plant volatile bouquets.Phytochemistry. 2011 Sep;72(13):1635-46. doi: 10.1016/j.phytochem.2011.01.019. Epub 2011 Feb 19. Phytochemistry. 2011. PMID: 21334702 Review.
-
Identification and functional analysis of two P450 enzymes of Gossypium hirsutum involved in DMNT and TMTT biosynthesis.Plant Biotechnol J. 2018 Feb;16(2):581-590. doi: 10.1111/pbi.12797. Epub 2017 Aug 16. Plant Biotechnol J. 2018. PMID: 28710782 Free PMC article.
-
Identification and regulation of TPS04/GES, an Arabidopsis geranyllinalool synthase catalyzing the first step in the formation of the insect-induced volatile C16-homoterpene TMTT.Plant Cell. 2008 Apr;20(4):1152-68. doi: 10.1105/tpc.106.049478. Epub 2008 Apr 8. Plant Cell. 2008. PMID: 18398052 Free PMC article.
-
Characterization of Biosynthetic Pathways for the Production of the Volatile Homoterpenes DMNT and TMTT in Zea mays.Plant Cell. 2016 Oct;28(10):2651-2665. doi: 10.1105/tpc.15.00919. Epub 2016 Sep 23. Plant Cell. 2016. PMID: 27662898 Free PMC article.
-
Indirect plant defense against insect herbivores: a review.Insect Sci. 2018 Feb;25(1):2-23. doi: 10.1111/1744-7917.12436. Epub 2017 Mar 20. Insect Sci. 2018. PMID: 28035791 Review.
Cited by
-
Identification of semiochemicals released by cotton, Gossypium hirsutum, upon infestation by the cotton aphid, Aphis gossypii.J Chem Ecol. 2011 Jul;37(7):741-50. doi: 10.1007/s10886-011-9980-x. Epub 2011 Jun 14. J Chem Ecol. 2011. PMID: 21671083
-
Scenarios of Genes-to-Terpenoids Network Led to the Identification of a Novel α/β-Farnesene/β-Ocimene Synthase in Camellia sinensis.Int J Mol Sci. 2020 Jan 19;21(2):655. doi: 10.3390/ijms21020655. Int J Mol Sci. 2020. PMID: 31963919 Free PMC article.
-
Identification and characterization of two P450 enzymes from Citrus sinensis involved in TMTT and DMNT biosyntheses and Asian citrus psyllid defense.Hortic Res. 2024 Apr 1;11(4):uhae037. doi: 10.1093/hr/uhae037. eCollection 2024 Apr. Hortic Res. 2024. PMID: 38617747 Free PMC article.
-
Spider mite egg extract modifies Arabidopsis response to future infestations.Sci Rep. 2021 Sep 6;11(1):17692. doi: 10.1038/s41598-021-97245-z. Sci Rep. 2021. PMID: 34489518 Free PMC article.
-
Phylogeny and Functional Differentiation of the Terpene Synthase Gene Family in Angiosperms with Emphasis on Rosa chinensis.Int J Mol Sci. 2025 Feb 27;26(5):2113. doi: 10.3390/ijms26052113. Int J Mol Sci. 2025. PMID: 40076733 Free PMC article.
References
-
- Kaiser R. In: Perfumes: Art, Science and Technology. Müller PM, Lamparsky D, editors. London: Elsevier Applied Science; 1991. pp. 213–250.
-
- Su J-W, Zeng J-P, Qin X-W, Ge F. Effect of needle damage on the release rate of Masson pine (Pinus massoniana) volatiles. J Plant Res. 2009;122:193–200. - PubMed
-
- Degenhardt J, Gershenzon J. Demonstration and characterization of (E)-nerolidol synthase from maize: A herbivore-inducible terpene synthase participating in (3E)-4,8-dimethyl-1,3,7-nonatriene biosynthesis. Planta. 2000;210:815–822. - PubMed
-
- Arimura G, et al. Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature. 2000;406:512–515. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

