Ca2+ signaling by plant Arabidopsis thaliana Pep peptides depends on AtPepR1, a receptor with guanylyl cyclase activity, and cGMP-activated Ca2+ channels
- PMID: 21088220
- PMCID: PMC3000296
- DOI: 10.1073/pnas.1000191107
Ca2+ signaling by plant Arabidopsis thaliana Pep peptides depends on AtPepR1, a receptor with guanylyl cyclase activity, and cGMP-activated Ca2+ channels
Abstract
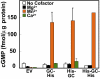
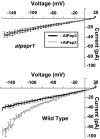
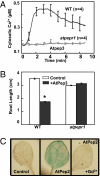
A family of peptide signaling molecules (AtPeps) and their plasma membrane receptor AtPepR1 are known to act in pathogen-defense signaling cascades in plants. Little is currently known about the molecular mechanisms that link these signaling peptides and their receptor, a leucine-rich repeat receptor-like kinase, to downstream pathogen-defense responses. We identify some cellular activities of these molecules that provide the context for a model for their action in signaling cascades. AtPeps activate plasma membrane inwardly conducting Ca(2+) permeable channels in mesophyll cells, resulting in cytosolic Ca(2+) elevation. This activity is dependent on their receptor as well as a cyclic nucleotide-gated channel (CNGC2). We also show that the leucine-rich repeat receptor-like kinase receptor AtPepR1 has guanylyl cyclase activity, generating cGMP from GTP, and that cGMP can activate CNGC2-dependent cytosolic Ca(2+) elevation. AtPep-dependent expression of pathogen-defense genes (PDF1.2, MPK3, and WRKY33) is mediated by the Ca(2+) signaling pathway associated with AtPep peptides and their receptor. The work presented here indicates that extracellular AtPeps, which can act as danger-associated molecular patterns, signal by interaction with their receptor, AtPepR1, a plasma membrane protein that can generate cGMP. Downstream from AtPep and AtPepR1 in a signaling cascade, the cGMP-activated channel CNGC2 is involved in AtPep- and AtPepR1-dependent inward Ca(2+) conductance and resulting cytosolic Ca(2+) elevation. The signaling cascade initiated by AtPeps leads to expression of pathogen-defense genes in a Ca(2+)-dependent manner.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Comment in
-
Guanylyl cyclase activity in plants?Proc Natl Acad Sci U S A. 2011 May 10;108(19):E96; author reply E97-8. doi: 10.1073/pnas.1101007108. Epub 2011 Apr 28. Proc Natl Acad Sci U S A. 2011. PMID: 21527716 Free PMC article. No abstract available.
References
-
- Baryshnikov SG, Pulina MV, Zulian A, Linde CI, Golovina VA. Orai1, a critical component of store-operated Ca2+ entry, is functionally associated with Na+/Ca2+ exchanger and plasma membrane Ca2+ pump in proliferating human arterial myocytes. Am J Physiol Cell Physiol. 2009;297:C1103–C1112. - PMC - PubMed
-
- Feske S, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. - PubMed
-
- Interlandi J. An immune portal. Sci Am. 2006;295:20–23. - PubMed
-
- Kaplan B, Sherman T, Fromm H. Cyclic nucleotide-gated channels in plants. FEBS Lett. 2007;581:2237–2246. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

