Local renal autoantibody production in lupus nephritis
- PMID: 21088295
- PMCID: PMC3029902
- DOI: 10.1681/ASN.2010050515
Local renal autoantibody production in lupus nephritis
Abstract
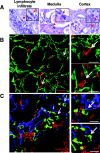
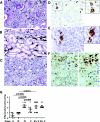
Autoantibodies are central to the pathogenesis of several autoimmune diseases including systemic lupus erythematosus. Plasma cells secrete these autoantibodies, but the anatomical sites of these cells are not well defined. Here, we found that although dsDNA-specific plasma cells in NZB/W mice were present in spleen and bone marrow, a large number were in the kidneys and their number correlated with the serum dsDNA-IgG titer. We observed renal plasma cells only in mice with nephritis, where they located mainly to the tubulointerstitium of the cortex and outer medulla. These cells had the phenotypic characteristics of fully differentiated plasma cells and, similar to long-lived bone marrow plasma cells, they were not in cell cycle. In patients with lupus nephritis, plasma cells were often present in the medulla in those with the most severe disease, especially combined proliferative and membranous lupus nephritis. The identification of the kidney as a major site of autoreactive plasma cells has implications for our understanding of the pathogenesis of lupus nephritis and for strategies to deplete autoreactive plasma cells, a long-standing therapeutic aim.
Figures


Similar articles
-
High frequency of autoantibody-secreting cells and long-lived plasma cells within inflamed kidneys of NZB/W F1 lupus mice.Eur J Immunol. 2011 Jul;41(7):2107-12. doi: 10.1002/eji.201041315. Epub 2011 Jun 6. Eur J Immunol. 2011. PMID: 21484784
-
Autoantibodies from long-lived 'memory' plasma cells of NZB/W mice drive immune complex nephritis.Ann Rheum Dis. 2013 Dec;72(12):2011-7. doi: 10.1136/annrheumdis-2013-203455. Epub 2013 Oct 10. Ann Rheum Dis. 2013. PMID: 24114925
-
Long-term B cell depletion in murine lupus eliminates autoantibody-secreting cells and is associated with alterations in the kidney plasma cell niche.J Immunol. 2014 Apr 1;192(7):3011-20. doi: 10.4049/jimmunol.1302003. Epub 2014 Feb 26. J Immunol. 2014. PMID: 24574498 Free PMC article.
-
Nuclease deficiencies promote end-stage lupus nephritis but not nephritogenic autoimmunity in (NZB × NZW) F1 mice.Immunol Cell Biol. 2011 Jan;89(1):90-9. doi: 10.1038/icb.2010.75. Epub 2010 Jun 15. Immunol Cell Biol. 2011. PMID: 20548325 Review.
-
Mechanisms of tissue injury in lupus nephritis.Arthritis Res Ther. 2011;13(6):250. doi: 10.1186/ar3528. Epub 2011 Dec 21. Arthritis Res Ther. 2011. PMID: 22192660 Free PMC article. Review.
Cited by
-
B Cell Recruitment Follows Kidney Injury and Maladaptive Repair.Transplantation. 2019 Aug;103(8):1527-1529. doi: 10.1097/TP.0000000000002723. Transplantation. 2019. PMID: 31348434 Free PMC article. No abstract available.
-
Naturally occurring autoimmune disease in (NZB X NZW) F1 mice is correlated with suppression of MZ B cell development due to aberrant B Cell Receptor (BCR) signaling, which is exacerbated by exposure to inorganic mercury.Toxicol Sci. 2023 Nov 11;197(2):211-21. doi: 10.1093/toxsci/kfad120. Online ahead of print. Toxicol Sci. 2023. PMID: 37952249 Free PMC article.
-
Dietary Docosahexaenoic Acid Prevents Silica-Induced Development of Pulmonary Ectopic Germinal Centers and Glomerulonephritis in the Lupus-Prone NZBWF1 Mouse.Front Immunol. 2018 Sep 12;9:2002. doi: 10.3389/fimmu.2018.02002. eCollection 2018. Front Immunol. 2018. PMID: 30258439 Free PMC article.
-
Targeting B and T Lymphocyte Attenuator Regulates Lupus Disease Development in NZB/W Mice.Immunotargets Ther. 2025 Jan 17;14:7-23. doi: 10.2147/ITT.S490573. eCollection 2025. Immunotargets Ther. 2025. PMID: 39845702 Free PMC article.
-
Guidelines for the use of flow cytometry and cell sorting in immunological studies.Eur J Immunol. 2017 Oct;47(10):1584-1797. doi: 10.1002/eji.201646632. Eur J Immunol. 2017. PMID: 29023707 Free PMC article. No abstract available.
References
-
- Fairhurst AM, Wandstrat AE, Wakeland EK: Systemic lupus erythematosus: Multiple immunological phenotypes in a complex genetic disease. Adv Immunol 92: 1–69, 2006 - PubMed
-
- Shlomchik MJ, Craft JE, Mamula MJ: From T to B and back again: Positive feedback in systemic autoimmune disease. Nat Rev Immunol 1: 147–153, 2001 - PubMed
-
- Dal Porto JM, Haberman AM, Shlomchik MJ, Kelsoe G: Antigen drives very low affinity B cells to become plasmacytes and enter germinal centers. J Immunol 161: 5373–5381, 1998 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

