Regulation of ΔNp63α by NFκΒ
- PMID: 21088498
- PMCID: PMC3047809
- DOI: 10.4161/cc.9.24.14093
Regulation of ΔNp63α by NFκΒ
Abstract
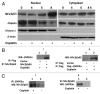
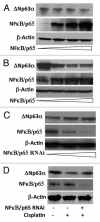
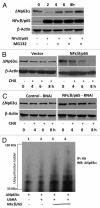
ΔNp63α, the dominant negative isoform of the p63 family is an essential survival factor in head and neck squamous cell carcinoma. This isoform has been shown to be down regulated in response to several DNA damaging agents, thereby enabling an effective cellular response to genotoxic agents. Here, we identify a key molecular mechanism underlying the regulation of ΔNp63α expression in response to extrinsic stimuli, such as chemotherapeutic agents. We show that ΔNp63α interacts with NF-κΒ in presence of cisplatin. We find that NF-κΒ promotes ubiquitin-mediated proteasomal degradation of ΔNp63α. Chemotherapy-induced stimulation of NF-κΒ leads to degradation of ΔNp63α and augments trans-activation of p53 family-induced genes involved in the cellular response to DNA damage. Conversely, inhibition of NF-κΒ with siRNA-mediated silencing NF-κΒ expression attenuates chemotherapy induced degradation of ΔNp63α . These data demonstrate that NF-κΒ plays an essential role in regulating ΔNp63α in response to extrinsic stimuli. Our findings suggest that the activation of NF-κΒ may be a mechanism by which levels of ΔNp63α are reduced, thereby rendering the cells susceptible to cell death in the face of cellular stress or DNA damage.
Figures

Similar articles
-
Regulation of p53 family member isoform DeltaNp63alpha by the nuclear factor-kappaB targeting kinase IkappaB kinase beta.Cancer Res. 2010 Feb 15;70(4):1419-29. doi: 10.1158/0008-5472.CAN-09-2613. Epub 2010 Feb 9. Cancer Res. 2010. PMID: 20145131 Free PMC article.
-
U-box-type ubiquitin E4 ligase, UFD2a attenuates cisplatin mediated degradation of DeltaNp63alpha.Cell Cycle. 2008 May 1;7(9):1231-7. doi: 10.4161/cc.7.9.5795. Epub 2008 Feb 19. Cell Cycle. 2008. PMID: 18418053 Free PMC article.
-
RACK1 and stratifin target DeltaNp63alpha for a proteasome degradation in head and neck squamous cell carcinoma cells upon DNA damage.Cell Cycle. 2004 Oct;3(10):1285-95. doi: 10.4161/cc.3.10.1155. Epub 2004 Oct 6. Cell Cycle. 2004. PMID: 15467455
-
Molecular Mechanisms of p63-Mediated Squamous Cancer Pathogenesis.Int J Mol Sci. 2019 Jul 23;20(14):3590. doi: 10.3390/ijms20143590. Int J Mol Sci. 2019. PMID: 31340447 Free PMC article. Review.
-
ΔNp63α in cancer: importance and therapeutic opportunities.Trends Cell Biol. 2023 Apr;33(4):280-292. doi: 10.1016/j.tcb.2022.08.003. Epub 2022 Sep 14. Trends Cell Biol. 2023. PMID: 36115734 Free PMC article. Review.
Cited by
-
p63 the guardian of human reproduction.Cell Cycle. 2012 Dec 15;11(24):4545-51. doi: 10.4161/cc.22819. Epub 2012 Nov 19. Cell Cycle. 2012. PMID: 23165243 Free PMC article. Review.
-
ASPP2 suppresses squamous cell carcinoma via RelA/p65-mediated repression of p63.Proc Natl Acad Sci U S A. 2013 Oct 29;110(44):17969-74. doi: 10.1073/pnas.1309362110. Epub 2013 Oct 14. Proc Natl Acad Sci U S A. 2013. PMID: 24127607 Free PMC article.
-
Silencing of high-mobility group box 2 (HMGB2) modulates cisplatin and 5-fluorouracil sensitivity in head and neck squamous cell carcinoma.Proteomics. 2015 Jan;15(2-3):383-93. doi: 10.1002/pmic.201400338. Proteomics. 2015. PMID: 25327479 Free PMC article.
-
Delineating Molecular Mechanisms of Squamous Tissue Homeostasis and Neoplasia: Focus on p63.J Skin Cancer. 2013;2013:632028. doi: 10.1155/2013/632028. Epub 2013 Apr 22. J Skin Cancer. 2013. PMID: 23710361 Free PMC article.
-
HSP90 Inhibitor SNX5422/2112 Targets the Dysregulated Signal and Transcription Factor Network and Malignant Phenotype of Head and Neck Squamous Cell Carcinoma.Transl Oncol. 2013 Aug 1;6(4):429-41. doi: 10.1593/tlo.13292. Print 2013 Aug. Transl Oncol. 2013. PMID: 23908686 Free PMC article.
References
-
- Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V, et al. p63, a p53 homolog at 3q27–29, encodes multiple products with transactivating, death-inducing and dominant-negative activities. Mol Cell. 1998;2:305–316. - PubMed
-
- Rocco JW, Leong CO, Kuperwasser N, DeYoung MP, Ellisen LW. p63 mediates survival in squamous cell carcinoma by suppression of p73-dependent apoptosis. Cancer Cell. 2006;9:45–56. - PubMed
-
- Benard J, Douc-Rasy S, Ahomadegbe JC. TP53 family members and human cancers. Hum Mutat. 2003;21:182–191. - PubMed
-
- Ishimoto O, Kawahara C, Enjo K, Obinata M, Nukiwa T, Ikawa S. Possible oncogenic potential of DeltaNp73: A newly identified isoform of human p73. Cancer Res. 2002;62:636–641. - PubMed
-
- Jost CA, Marin MC, Kaelin WG., Jr p73 is a simian [correction of human] p53-related protein that can induce apoptosis. Nature. 1997;389:191–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
