Sterile inflammation: sensing and reacting to damage
- PMID: 21088683
- PMCID: PMC3114424
- DOI: 10.1038/nri2873
Sterile inflammation: sensing and reacting to damage
Abstract
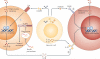
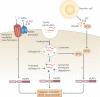
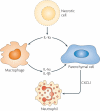
Over the past several decades, much has been revealed about the nature of the host innate immune response to microorganisms, with the identification of pattern recognition receptors (PRRs) and pathogen-associated molecular patterns, which are the conserved microbial motifs sensed by these receptors. It is now apparent that these same PRRs can also be activated by non-microbial signals, many of which are considered as damage-associated molecular patterns. The sterile inflammation that ensues either resolves the initial insult or leads to disease. Here, we review the triggers and receptor pathways that result in sterile inflammation and its impact on human health.
Figures
Comment in
-
Heat shock proteins are no DAMPs, rather 'DAMPERs'.Nat Rev Immunol. 2011 Jul 25;11(8):565; author reply 565. doi: 10.1038/nri2873-c1. Nat Rev Immunol. 2011. PMID: 21785457 No abstract available.
References
-
- Mossman BT, Churg A. Mechanisms in the pathogenesis of asbestosis and silicosis. Am. J. Respir. Crit. Care Med. 1998;157:1666–1680. - PubMed
-
- Cotran RS, Kumar V, Robbins S. In: Robbins Pathologic Basis of Disease. Schoen FJ, editor. W. B. Saunders Company; Philadelphia: 1994. pp. 6–11.
-
- Cotran RS, Kumar V, Robbins S. In: Robbins Pathologic Basis of Disease. Schoen FJ, editor. W. B. Saunders Company; Philadelphia: 1994. pp. 1255–1259.
-
- Weiner HL, Frenkel D. Immunology and immunotherapy of Alzheimer's disease. Nature Rev. Immunol. 2006;6:404–416. - PubMed
-
- Ross R. Atherosclerosis — an inflammatory disease. N. Engl. J. Med. 1999;340:115–126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

