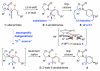
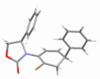
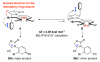
Stereoselective 6π-electron electrocyclic ring closures of 2-halo-amidotrienes via a remote 1,6-asymmetric induction
- PMID: 21090590
- PMCID: PMC3010762
- DOI: 10.1021/ol102693e
Stereoselective 6π-electron electrocyclic ring closures of 2-halo-amidotrienes via a remote 1,6-asymmetric induction
Abstract
A diastereoselective 6π-electrocyclic ring closure employing halogen-substituted 3-amidotrienes via a 1,6-remote asymmetric induction is described. This new asymmetric manifold for pericyclic ring closure further underscores the significance of the allenamide chemistry.
Figures
Similar articles
-
A tandem 1,3-H-shift-6π-electrocyclization-cyclic 2-amido-diene intramolecular Diels-Alder cycloaddition approach to BCD-Ring of atropurpuran.Org Lett. 2012 Jan 6;14(1):252-5. doi: 10.1021/ol203030a. Epub 2011 Dec 13. Org Lett. 2012. PMID: 22149386 Free PMC article.
-
Stereoselective formal [3 + 3] cycloaddition approach to cis-1-azadecalins and synthesis of (-)-4a,8a-diepi-pumiliotoxin C. evidence for the first highly stereoselective 6pi-electron electrocyclic ring closures of 1-azatrienes.J Am Chem Soc. 2002 Sep 4;124(35):10435-42. doi: 10.1021/ja020698b. J Am Chem Soc. 2002. PMID: 12197745
-
Total syntheses of (+/-)-cis-trikentrin A and (+/-)-cis-trikentrin B via electrocyclic ring closures of 2,3-divinylpyrrolines.Org Lett. 2006 Jul 20;8(15):3403-6. doi: 10.1021/ol061259a. Org Lett. 2006. PMID: 16836416
-
[Synthetic Studies of Bioactive Heterocyclic Natural Products and Fused Heterocyclic Compounds Based on the Thermal Electrocyclic or Azaelectocyclic Reaction of 6π-Electron or Aza-6π-electron Systems].Yakugaku Zasshi. 2016;136(4):607-48. doi: 10.1248/yakushi.15-00273. Yakugaku Zasshi. 2016. PMID: 27040345 Review. Japanese.
-
[Novel Methods for the Synthesis of Heterocycles Using Highly Reactive Spirocyclopropanes].Yakugaku Zasshi. 2018;138(1):19-25. doi: 10.1248/yakushi.17-00188. Yakugaku Zasshi. 2018. PMID: 29311461 Review. Japanese.
Cited by
-
An efficient and practical entry to 2-amido-dienes and 3-amido-trienes from allenamides through stereoselective 1,3-hydrogen shifts.Beilstein J Org Chem. 2011 Apr 7;7:410-20. doi: 10.3762/bjoc.7.53. Beilstein J Org Chem. 2011. PMID: 21512601 Free PMC article.
-
Facile Synthesis of 3-Amido-Dienynes via a Tandem α-Propargylation-Isomerization of Chiral Allenamides and their Applications in Diels-Alder Cycloadditions.Synlett. 2017 Dec;28(20):2906-2912. doi: 10.1055/s-0036-1590899. Epub 2017 Sep 1. Synlett. 2017. PMID: 29910538 Free PMC article.
-
Stereoselective synthesis of isoquinuclidines through an aza-[4 + 2] cycloaddition of chiral cyclic 2-amidodienes.Org Lett. 2014 Mar 21;16(6):1826-9. doi: 10.1021/ol500390a. Epub 2014 Mar 12. Org Lett. 2014. PMID: 24621094 Free PMC article.
-
A tandem 1,3-H-shift-6π-electrocyclization-cyclic 2-amido-diene intramolecular Diels-Alder cycloaddition approach to BCD-Ring of atropurpuran.Org Lett. 2012 Jan 6;14(1):252-5. doi: 10.1021/ol203030a. Epub 2011 Dec 13. Org Lett. 2012. PMID: 22149386 Free PMC article.
-
Oppolzer-type intramolecular Diels-Alder cycloadditions via isomerizations of allenamides.Org Lett. 2011 Jun 17;13(12):3114-7. doi: 10.1021/ol2010227. Epub 2011 May 25. Org Lett. 2011. PMID: 21612235 Free PMC article.
References
-
-
For reviews for pericyclic ring-closures, see: Marvell EN. Thermal Electrocyclic Reactions. Academic Press; New York: 1980. Okamura WH, de Lera AR. In: Comprehensive Organic Synthesis. Trost BM, Fleming I, Paquette LA, editors. Vol. 5. Pergamon Press; New York: 1991. pp. 699–750. For reviews on ring-closure in natural product synthesis, see: Pindur U, Schneider GH. Chem. Soc. Rev. 1994:409. Beaudry CM, Malerich JP, Trauner D. Chem. Rev. 2005;105:4757.
-
-
-
For recent examples of 6-π-electron electrocyclic ring-closures of 1,3,5-hexatrienes, see: Bishop LM, Barbarow JE, Bergmen RG, Trauner D. Angew. Chem. Int. Ed. Engl. 2008;47:8100. Sofiyev V, Navarro G, Trauner D. Org. Lett. 2008;10:149. Kan SBJ, Anderson EA. Org. Lett. 2008;10:2323. Hulot C, Blong G, Suffert J. J. Am. Chem. Soc. 2008;130:5046. Benson CL, West FG. Org. Lett. 2007;9:2545. Pouwer RH, Schill H, Williams CM, Bernhardt PV. Eur. J. Org. Chem. 2007:4699. Jung ME, Min S,-J. Tetrahedron. 2007;63:3682. For examples on accelerated ring-closures of 1,3,5-hexatrienes, see: Sünnemann HW, Banwell MG, de Meijere A. Eur. J. Org. Chem. 2007:3879. Tessier PE, Nguyen N, Clay MD, Fallis AG. J. Am. Chem. Soc. 2006;128:4946. Huntley RJ, Funk RL. Org. Lett. 2006;8:3403. Yu T-Q, Fu Y, Liu L, Guo Q-X. J. Org. Chem. 2006;71:6157. Magomedov NA, Ruggiero PL, Tang Y. J. Am. Chem. Soc. 2004;126:1624.
-
-
-
For a leading review on allenamide chemistry, see: Hsung RP, Wei L-L, Xiong H. Acc. Chem. Res. 2003;36:773. For general reviews on allenes, see: Krause N, Hashmi ASK. Modern Allene Chemistry. Vol 1 and 2. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim: 2004.
-
-
-
Given the large volume of recent activities in allenamide chemistry, for reports in 2009 and 2010, see: Lohse AG, Krenske EH, Antoline JE, Houk KN, Hsung RP. Org. Lett. 2010 ASAP. Beccalli EM, Bernasconi A, Borsini E, Broggini G, Rigamonti M, Zecchi G. J. Org. Chem. 2010;75:6923. Hill AW, Elsegood MRJ, Kimber MC. J. Org. Chem. 2010;75:5406. Persson AKÅ, Bäckvall J-E. Angew. Chem. Int. Ed. 2010;49:4624. Krenske EK, Houk KN, Lohse AG, Antoline JE, Hsung RP. Chem. Science. 2010;1:387. Danowitz AM, Taylor CE, Shrikian TM, Mapp AK. Org. Lett. 2010;12:2574. Zbieg JR, E., McInturff EL, Krische MJ. Org. Lett. 2010;12:2514. Cordier P, Aubert C, Malacria M, Gandon V, Lacôte E. Chem. Eur. J. 2010;16:9973. Kimber MC. Org. Lett. 2010;12:1128. Hashimoto K, Horino Y, Kuroda S. Heterocycles. 2010;80:187. Persson AKÅ, Johnston EV, Bäckvall J-E. Org. Lett. 2009;11:3814. Skucas E, Zbieg JR, Krische MJ. J. Am. Chem. Soc. 2009;131:5054. Armstrong A, Emmerson DPG. Org. Lett. 2009;11:1547. Beccalli EM, Broggini G, Clerici F, Galli S, Kammerer C, Rigamonti M, Sottocornola S. Org. Lett. 2009;11:1563. Broggini G, Galli S, Rigamonti M, Sottocornola S, Zecchi G. Tetrahedron Lett. 2009;50:1447. Lohse AG, Hsung RP. Org. Lett. 2009;11:3430. Lu T, Hayashi R, Hsung RP, DeKorver KA, Lohse AG, Song Z, Tang Y. Org. Biomol. Chem. 2009;9:3331.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources