Recent advances in colloidal and interfacial phenomena involving liquid crystals
- PMID: 21090596
- PMCID: PMC3089817
- DOI: 10.1021/la103301d
Recent advances in colloidal and interfacial phenomena involving liquid crystals
Abstract
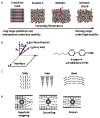
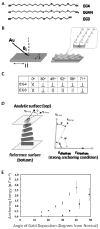
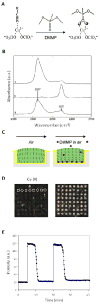
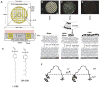
This feature article describes recent advances in several areas of research involving the interfacial ordering of liquid crystals (LCs). The first advance revolves around the ordering of LCs at bio/chemically functionalized surfaces. Whereas the majority of past studies of surface-induced ordering of LCs have involved surfaces of solids that present a limited diversity of chemical functional groups (surfaces at which van der Waals forces dominate surface-induced ordering), recent studies have moved to investigate the ordering of LCs on chemically complex surfaces. For example, surfaces decorated with biomolecules (e.g., oligopeptides and proteins) and transition-metal ions have been investigated, leading to an understanding of the roles that metal-ligand coordination interactions, electrical double layers, acid-base interactions, and hydrogen bonding can play in the interfacial ordering of LCs. The opportunity to create chemically responsive LCs capable of undergoing ordering transitions in the presence of targeted molecular events (e.g., ligand exchange around a metal center) has emerged from these fundamental studies. A second advance has focused on investigations of the ordering of LCs at interfaces with immiscible isotropic fluids, particularly water. In contrast to prior studies of surface-induced ordering of LCs on solid surfaces, LC-aqueous interfaces are deformable and molecules at these interfaces exhibit high levels of mobility and thus can reorganize in response to changes in the interfacial environment. A range of fundamental investigations involving these LC-aqueous interfaces have revealed that (i) the spatial and temporal characteristics of assemblies formed from biomolecular interactions can be reported by surface-driven ordering transitions in the LCs, (ii) the interfacial phase behavior of molecules and colloids can be coupled to (and manipulated via) the ordering (and nematic elasticity) of LCs, and (iii) the confinement of LCs leads to unanticipated size-dependent ordering (particularly in the context of LC emulsion droplets). The third and final advance addressed in this article involves interactions between colloids mediated by LCs. Recent experiments involving microparticles deposited at the LC-aqueous interface have revealed that LC-mediated interactions can drive interfacial assemblies of particles through reversible ordering transitions (e.g., from 1D chains to 2D arrays with local hexagonal symmetry). In addition, recent single-nanoparticle measurements suggest that the ordering of LCs about nanoparticles differs substantially from micrometer-sized particles and that the interactions between nanoparticles mediated by the LCs are far weaker than predicted by theory (sufficiently weak that the interactions are reversible and thus enable self-assembly). Finally, LC-mediated interactions between colloidal particles have also been shown to lead to the formation of colloid-in-LC gels that possess mechanical properties relevant to the design of materials that interface with living biological systems. Overall, these three topics serve to illustrate the broad opportunities that exist to do fundamental interfacial science and discovery-oriented research involving LCs.
Figures






Similar articles
-
Chemoresponsive assemblies of microparticles at liquid crystalline interfaces.Proc Natl Acad Sci U S A. 2010 Mar 2;107(9):3998-4003. doi: 10.1073/pnas.0910931107. Epub 2010 Jan 26. Proc Natl Acad Sci U S A. 2010. PMID: 20133750 Free PMC article.
-
Design of Responsive and Active (Soft) Materials Using Liquid Crystals.Annu Rev Chem Biomol Eng. 2016 Jun 7;7:163-96. doi: 10.1146/annurev-chembioeng-061114-123323. Epub 2016 Mar 10. Annu Rev Chem Biomol Eng. 2016. PMID: 26979412 Free PMC article. Review.
-
Colloid-in-liquid crystal gels that respond to biomolecular interactions.Small. 2013 Aug 26;9(16):2785-92, 2784. doi: 10.1002/smll.201202869. Epub 2013 Apr 2. Small. 2013. PMID: 23554243 Free PMC article.
-
Ordering transitions in nematic liquid crystals induced by vesicles captured through ligand-receptor interactions.Langmuir. 2011 Feb 15;27(4):1419-29. doi: 10.1021/la103975s. Epub 2010 Dec 13. Langmuir. 2011. PMID: 21142099 Free PMC article.
-
Introduction to optical methods for characterizing liquid crystals at interfaces.Langmuir. 2013 Mar 12;29(10):3154-69. doi: 10.1021/la304679f. Epub 2013 Feb 26. Langmuir. 2013. PMID: 23347378 Free PMC article. Review.
Cited by
-
Liquid Crystal@Nanosilver Catalytic Amplification-Aptamer Trimode Biosensor for Trace Pb2.Int J Mol Sci. 2023 Feb 2;24(3):2920. doi: 10.3390/ijms24032920. Int J Mol Sci. 2023. PMID: 36769237 Free PMC article.
-
Improving liquid-crystal-based biosensing in aqueous phases.ACS Appl Mater Interfaces. 2012 Dec;4(12):6884-90. doi: 10.1021/am301952f. Epub 2012 Dec 12. ACS Appl Mater Interfaces. 2012. PMID: 23157269 Free PMC article.
-
Fibonacci, quasicrystals and the beauty of flowers.Plant Signal Behav. 2012 Dec;7(12):1721-3. doi: 10.4161/psb.22417. Epub 2012 Oct 16. Plant Signal Behav. 2012. PMID: 23072998 Free PMC article.
-
Chiral Liquid Crystal Microdroplets for Sensing Phospholipid Amphiphiles.Biosensors (Basel). 2022 May 9;12(5):313. doi: 10.3390/bios12050313. Biosensors (Basel). 2022. PMID: 35624614 Free PMC article.
-
Liquid Crystal-Templated Porous Microparticles via Photopolymerization of Temperature-Induced Droplets in a Binary Liquid Mixture.ACS Omega. 2023 May 30;8(23):20404-20411. doi: 10.1021/acsomega.3c00490. eCollection 2023 Jun 13. ACS Omega. 2023. PMID: 37323413 Free PMC article.
References
-
- de Gennes PG, Prost J. The Physics of Liquid Crystals. Oxford University Press; London: 1994.
-
- Collings PJ, Hird M. Introduction to Liquid Crystals. Taylor & Francis Ltd; 1997.
-
- Jerome B. Rep Prog Phys. 1991;54:391–451.
-
- Gupta VK, Abbott NL. Physical Review E. 1996;54:4540–4543. - PubMed
-
- Lockwood NA, Gupta JK, Abbott NL. Surface Science Reports. 2008;63:255–293.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

