High-throughput metabolic toxicity screening using magnetic biocolloid reactors and LC-MS/MS
- PMID: 21090635
- PMCID: PMC3005372
- DOI: 10.1021/ac102317a
High-throughput metabolic toxicity screening using magnetic biocolloid reactors and LC-MS/MS
Abstract
An inexpensive, high-throughput genotoxicity screening method was developed by using magnetic particles coated with cytosol/microsome/DNA films as biocolloid reactors in a 96-well plate format coupled with liquid chromatography-mass spectrometry. Incorporation of both microsomal and cytosolic enzymes in the films provides a broad spectrum of metabolic enzymes representing a range of metabolic pathways for bioactivation of chemicals. Reactive metabolites generated via this process are trapped by covalently binding to DNA in the film. The DNA is then hydrolyzed and nucleobase adducts are collected using filters in the bottom for the 96-well plate of analysis by capillary liquid chromatography-tandem mass spectrometry (LC-MS/MS). The magnetic particles facilitate simple and rapid sample preparation and workup. Major DNA adducts from ethylene dibromide, N-acetyl-2-aminofluorene and styrene were identified in proof-of-concept studies. Relative formation rates of DNA adducts correlated well with rodent genotoxicity metric TD(50) for the three compounds. This method has the potential for high-throughput genotoxicity screening, providing chemical structure information that is complementary to toxicity bioassays.
Figures

 ), area ratio to internal standard 7-methylguanosine (m/z 298>166).
), area ratio to internal standard 7-methylguanosine (m/z 298>166).



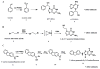
References
-
- Apruzzese WA, Vouros P. J Chromatogr A. 1998;794:97–108. - PubMed
-
- Koc H, Swenberg JA. J Chromatogr B. 2002;778:323–343. - PubMed
- Farmer PB, Singh R. Mutat Res, Rev Mutat Res. 2008;659:68–76. - PubMed
- La DK, Swenberg JA. Mutat Res, Genet Toxicol. 1996;365:129–146. - PubMed
- Farmer PB, Brown K, Tompkins E, Emms VL, Jones DJL, Singh R, Phillips DH. Toxicol Appl Pharmacol. 2005;207:293–301. - PubMed
-
- Tarun M, Rusling JF. Crit Rev Eukaryot Gene Expr. 2005;15:295–315. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

