Preexposure chemoprophylaxis for HIV prevention in men who have sex with men
- PMID: 21091279
- PMCID: PMC3079639
- DOI: 10.1056/NEJMoa1011205
Preexposure chemoprophylaxis for HIV prevention in men who have sex with men
Abstract
Background: Antiretroviral chemoprophylaxis before exposure is a promising approach for the prevention of human immunodeficiency virus (HIV) acquisition.
Methods: We randomly assigned 2499 HIV-seronegative men or transgender women who have sex with men to receive a combination of two oral antiretroviral drugs, emtricitabine and tenofovir disoproxil fumarate (FTC-TDF), or placebo once daily. All subjects received HIV testing, risk-reduction counseling, condoms, and management of sexually transmitted infections.
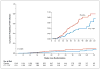
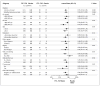
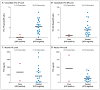
Results: The study subjects were followed for 3324 person-years (median, 1.2 years; maximum, 2.8 years). Of these subjects, 10 were found to have been infected with HIV at enrollment, and 100 became infected during follow-up (36 in the FTC-TDF group and 64 in the placebo group), indicating a 44% reduction in the incidence of HIV (95% confidence interval, 15 to 63; P=0.005). In the FTC-TDF group, the study drug was detected in 22 of 43 of seronegative subjects (51%) and in 3 of 34 HIV-infected subjects (9%) (P<0.001). Nausea was reported more frequently during the first 4 weeks in the FTC-TDF group than in the placebo group (P<0.001). The two groups had similar rates of serious adverse events (P=0.57).
Conclusions: Oral FTC-TDF provided protection against the acquisition of HIV infection among the subjects. Detectable blood levels strongly correlated with the prophylactic effect. (Funded by the National Institutes of Health and the Bill and Melinda Gates Foundation; ClinicalTrials.gov number, NCT00458393.).
Figures
Comment in
-
Oral preexposure prophylaxis for HIV--another arrow in the quiver?N Engl J Med. 2010 Dec 30;363(27):2663-5. doi: 10.1056/NEJMe1012929. Epub 2010 Nov 23. N Engl J Med. 2010. PMID: 21091280 No abstract available.
-
Preexposure chemoprophylaxis for HIV prevention.N Engl J Med. 2011 Apr 7;364(14):1374; author reply 1374-5. doi: 10.1056/NEJMc1101504. N Engl J Med. 2011. PMID: 21470022 No abstract available.
-
Preexposure chemoprophylaxis for HIV prevention.N Engl J Med. 2011 Apr 7;364(14):1374; author reply 1374-5. doi: 10.1056/NEJMc1101504. N Engl J Med. 2011. PMID: 21470023 No abstract available.
-
Preexposure chemoprophylaxis for HIV prevention.N Engl J Med. 2011 Apr 7;364(14):1373; author reply 1374-5. doi: 10.1056/NEJMc1101504. N Engl J Med. 2011. PMID: 21470024 No abstract available.
-
Preexposure chemoprophylaxis for HIV prevention.N Engl J Med. 2011 Apr 7;364(14):1373; author reply 1374-5. doi: 10.1056/NEJMc1101504. N Engl J Med. 2011. PMID: 21470025 No abstract available.
-
Preexposure chemoprophylaxis for HIV prevention.N Engl J Med. 2011 Apr 7;364(14):1372-3; author reply 1374-5. doi: 10.1056/NEJMc1101504. N Engl J Med. 2011. PMID: 21470026 No abstract available.
-
ACP Journal Club. Daily emtricitabine plus tenofovir disoproxil fumarate reduced risk for HIV infection in men who have sex with men.Ann Intern Med. 2011 Apr 19;154(8):JC4-2. doi: 10.7326/0003-4819-154-8-201104190-02002. Ann Intern Med. 2011. PMID: 21502640 No abstract available.
-
Oral antiretroviral pre-exposure prophylaxis reduces the risk of HIV acquisition among men who have sex with men.Evid Based Med. 2011 Oct;16(5):146-7. doi: 10.1136/ebm1305. Epub 2011 May 10. Evid Based Med. 2011. PMID: 21558562 Free PMC article. No abstract available.
-
[Antiretroviral prophylaxis before exposure for men with HIV contamination risk?].Med Mal Infect. 2011 Aug;41(8):449. doi: 10.1016/j.medmal.2011.03.006. Med Mal Infect. 2011. PMID: 21936070 French. No abstract available.
-
Re.: Preexposure chemoprophylaxis for HIV prevention in men who have sex with men.J Urol. 2011 May;185(5):1729-30. doi: 10.1016/S0022-5347(11)60198-5. J Urol. 2011. PMID: 22088703 No abstract available.
References
-
- Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. N Engl J Med. 1994;331:1173–80. - PubMed
-
- Smith DK, Grohskopf LA, Black RJ, et al. Antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposure to HIV in the United States: recommendations from the U.S. Department of Health and Human Services. MMWR Recomm Rep. 2005;54(RR-2):1–20. - PubMed
-
- Schechter M, do Lago RF, Mendelsohn AB, Moreira RI, Moulton LH, Harrison LH. Behavioral impact, acceptability, and HIV incidence among homosexual men with access to postexposure chemoprophylaxis for HIV. J Acquir Immune Defic Syndr. 2004;35:519–25. - PubMed
-
- Roland ME, Neilands TB, Krone MR, et al. Seroconversion following nonoccupational postexposure prophylaxis against HIV. Clin Infect Dis. 2005;41:1507–13. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
