Mechanisms of gender-specific regulation of mouse sulfotransferases (Sults)
- PMID: 21091322
- PMCID: PMC5506857
- DOI: 10.3109/00498254.2010.535923
Mechanisms of gender-specific regulation of mouse sulfotransferases (Sults)
Abstract
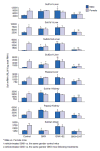
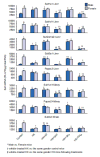
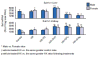
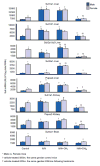
1. Marked gender differences in the expression of sulfotransferases (Sults) are known to exist in several species including rats, mice and hamsters. However, the mechanism for this gender difference is not known. Therefore, in the present study, it was determined whether sex and/or growth hormone (GH) are responsible for the gender difference in the expression of Sults using gonadectomized (GNX), hypophysectomized (HX) and GH-releasing hormone receptor-deficient little (lit/lit) mouse models. 2. Sult1a1 and Papss2 in liver and kidney, and Sult1d1 in liver are female-predominant in mice because of suppressive effects of both androgens and male-pattern GH secretion. Sult2a1/a2 is the most markedly female-predominant Sult in mouse liver due to suppressive effects of androgens and male-pattern GH secretion, as well as stimulatory effects by estrogens and female-pattern GH secretion. Sult3a1 is female-predominant in mouse liver due to suppressive effects of androgens as well as stimulatory effects of estrogens and female-pattern GH secretion. Sult1c1 expression is male-predominant in mouse liver and kidney because of stimulatory effects of androgens in males. Sult4a1 expression is female-predominant in mouse brain due to stimulatory effects of estrogens. 3. In conclusion, gender-divergent Sults are mostly female-predominant and Sult1c1 is the only male-dominant Sult. The gender differences in expression of various mouse Sults are influenced by various mechanisms involving sex and/or GHs.
Conflict of interest statement
This work was supported by NIH grants ES-09649, ES-09716, ES013714 and COBRE grant P20-RR-021940.
Figures


References
-
- Ahluwalia A, Clodfelter KH, Waxman DJ. Sexual dimorphism f rat liver gene expression: regulatory role of growth hormone revealed by deoxyribonucleic Acid microarray analysis. Mol Endocrinol. 2004;18:747–760. - PubMed
-
- Aksoy IA, Sochorová V, Weinshilboum RM. Human liver dehydroepiandrosterone sulfotransferase: nature and extent of individual variation. Clin Pharmacol Ther. 1993;54:498–506. - PubMed
-
- Alnouti Y, Klaassen CD. Tissue distribution and ontogeny of sulfotransferase enzymes in mice. Toxicol Sci. 2006;93:242–255. - PubMed
-
- Alnouti Y, Klaassen CD. Regulation of sulfotransferase enzymes by prototypical microsomal enzyme inducers in mice. J Pharmacol Exp Ther. 2008;324:612–621. - PubMed
-
- Balistreri WF, Zimmer L, Suchy FJ, Bove KE. Bile salt sulfotransferase: alterations during maturation and non-inducibility during substrate ingestion. J Lipid Res. 1984;25:228–235. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
