Fibrocartilage tissue engineering: the role of the stress environment on cell morphology and matrix expression
- PMID: 21091338
- PMCID: PMC3117236
- DOI: 10.1089/ten.TEA.2009.0499
Fibrocartilage tissue engineering: the role of the stress environment on cell morphology and matrix expression
Abstract
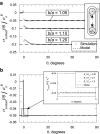
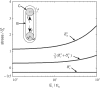
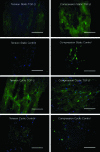
Although much is known about the effects of uniaxial mechanical loading on fibrocartilage development, the stress fields to which fibrocartilaginous regions are subjected to during development are mutiaxial. That fibrocartilage develops at tendon-to-bone attachments and in compressive regions of tendons is well established. However, the three-dimensional (3D) nature of the stresses needed for the development of fibrocartilage is not known. Here, we developed and applied an in vitro system to determine whether fibrocartilage can develop under a state of periodic hydrostatic tension in which only a single principal component of stress is compressive. This question is vital to efforts to mechanically guide morphogenesis and matrix expression in engineered tissue replacements. Mesenchymal stromal cells in a 3D culture were exposed to compressive and tensile stresses as a result of an external tensile hydrostatic stress field. The stress field was characterized through mechanical modeling. Tensile cyclic stresses promoted spindle-shaped cells, upregulation of scleraxis and type one collagen, and cell alignment with the direction of tension. Cells experiencing a single compressive stress component exhibited rounded cell morphology and random cell orientation. No difference in mRNA expression of the genes Sox9 and aggrecan was observed when comparing tensile and compressive regions unless the medium was supplemented with the chondrogenic factor transforming growth factor beta3. In that case, Sox9 was upregulated under static loading conditions and aggrecan was upregulated under cyclic loading conditions. In conclusion, the fibrous component of fibrocartilage could be generated using only mechanical cues, but generation of the cartilaginous component of fibrocartilage required biologic factors in addition to mechanical cues. These studies support the hypothesis that the 3D stress environment influences cell activity and gene expression in fibrocartilage development.
Figures






Similar articles
-
Tensile loading modulates bone marrow stromal cell differentiation and the development of engineered fibrocartilage constructs.Tissue Eng Part A. 2010 Jun;16(6):1913-23. doi: 10.1089/ten.TEA.2009.0561. Tissue Eng Part A. 2010. PMID: 20088686 Free PMC article.
-
Dynamic tensile loading improves the functional properties of mesenchymal stem cell-laden nanofiber-based fibrocartilage.Tissue Eng Part A. 2011 May;17(9-10):1445-55. doi: 10.1089/ten.TEA.2010.0535. Epub 2011 Mar 3. Tissue Eng Part A. 2011. PMID: 21247342 Free PMC article.
-
Mechanoactive scaffold induces tendon remodeling and expression of fibrocartilage markers.Clin Orthop Relat Res. 2008 Aug;466(8):1938-48. doi: 10.1007/s11999-008-0310-8. Epub 2008 May 30. Clin Orthop Relat Res. 2008. PMID: 18512112 Free PMC article.
-
Molecular parameters indicating adaptation to mechanical stress in fibrous connective tissue.Adv Anat Embryol Cell Biol. 2005;178:1-71. Adv Anat Embryol Cell Biol. 2005. PMID: 16080262 Review.
-
Fibrocartilage in tendons and ligaments--an adaptation to compressive load.J Anat. 1998 Nov;193 ( Pt 4)(Pt 4):481-94. doi: 10.1046/j.1469-7580.1998.19340481.x. J Anat. 1998. PMID: 10029181 Free PMC article. Review.
Cited by
-
Scaffold-free cell-based tissue engineering therapies: advances, shortfalls and forecast.NPJ Regen Med. 2021 Mar 29;6(1):18. doi: 10.1038/s41536-021-00133-3. NPJ Regen Med. 2021. PMID: 33782415 Free PMC article. Review.
-
Loss of scleraxis in mice leads to geometric and structural changes in cortical bone, as well as asymmetry in fracture healing.FASEB J. 2017 Mar;31(3):882-892. doi: 10.1096/fj.201600969R. Epub 2016 Nov 18. FASEB J. 2017. PMID: 27864378 Free PMC article.
-
The role of mechanics in actin stress fiber kinetics.Exp Cell Res. 2013 Oct 1;319(16):2490-500. doi: 10.1016/j.yexcr.2013.06.017. Epub 2013 Jul 29. Exp Cell Res. 2013. PMID: 23906923 Free PMC article. Review.
-
Effect of micro-strain stress on in vitro proliferation and functional expression of human osteoarthritic chondrocytes.J Orthop Surg Res. 2022 Feb 15;17(1):93. doi: 10.1186/s13018-022-02987-9. J Orthop Surg Res. 2022. PMID: 35168651 Free PMC article.
-
Gradient bimetallic ion-based hydrogels for tissue microstructure reconstruction of tendon-to-bone insertion.Sci Adv. 2021 Jun 23;7(26):eabg3816. doi: 10.1126/sciadv.abg3816. Print 2021 Jun. Sci Adv. 2021. PMID: 34162547 Free PMC article.
References
-
- Praemer A. Furner S. Rice D. Musculoskeletal Conditions in the US. Park Ridge, IL: American Academy of Orthopaedic Surgeons; 1992.
-
- Rodeo S.A. Arnoczky S.P. Torzilli P.A. Hidaka C. Warren R.F. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75:1795. - PubMed
-
- McAndrews P.T. Arnoczky S.P. Meniscal repair enhancement techniques. Clin Sports Med. 1996;15:499. - PubMed
-
- Thomopoulos S. Williams G.R. Soslowsky L.J. Tendon to bone healing: differences in biomechanical, structural, and compositional properties due to a range of activity levels. J Biomech Eng. 2003;125:106. - PubMed
-
- Thomopoulos S. Marquez J.P. Weinberger B. Birman V. Genin G.M. Collagen fiber orientation at the tendon to bone insertion and its influence on stress concentrations. J Biomech. 2006;39:1842. - PubMed
Appendix References
-
- Jones R.M. Mechanics of Composite Materials. Philadelphia: Taylor & Francis, Inc; 1999.
-
- Lekhnitskii S.G. Anisotropic Plates. New York: Gordon and Breach; 1968.
-
- Saada A.S. Elasticity: Theory and Applications. Melbourne, FL: Krieger Publishing; 1993.
-
- Fosdick R. Royer-Carfagni G. The constraint of local injectivity in linear elasticity theory. Proc R Soc Lond A. 2001;457:2167.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

