CDC25B associates with a centrin 2-containing complex and is involved in maintaining centrosome integrity
- PMID: 21091437
- PMCID: PMC3025493
- DOI: 10.1042/BC20100111
CDC25B associates with a centrin 2-containing complex and is involved in maintaining centrosome integrity
Abstract
Background information: CDC25 (cell division cycle 25) phosphatases function as activators of CDK (cyclin-dependent kinase)-cyclin complexes to regulate progression through the CDC. We have recently identified a pool of CDC25B at the centrosome of interphase cells that plays a role in regulating centrosome numbers.
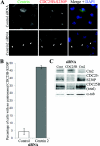
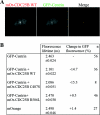
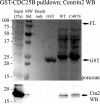
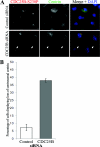
Results: In the present study, we demonstrate that CDC25B forms a close association with Ctn (centrin) proteins at the centrosome. This interaction involves both N- and C-terminal regions of CDC25B and requires CDC25B binding to its CDK-cyclin substrates. However, the interaction is not dependent on the enzyme activity of CDC25B. Although CDC25B appears to bind indirectly to Ctn2, this association is pertinent to CDC25B localization at the centrosome. We further demonstrate that CDC25B plays a role in maintaining the overall integrity of the centrosome, by regulating the centrosome levels of multiple centrosome proteins, including that of Ctn2.
Conclusions: Our results therefore suggest that CDC25B associates with a Ctn2-containing multiprotein complex in the cytoplasm, which targets it to the centrosome, where it plays a role in maintaining the centrosome levels of Ctn2 and a number of other centrosome components.
Figures

References
-
- Araki M., Masutani C., Takemura M., Uchida A., Sugasawa K., Kondoh J., Ohkuma Y., Hanaoka F. Centrosome protein centrin 2/caltractin 1 is part of the xeroderma pigmentosum group C complex that initiates global genome nucleotide excision repair. J. Biol. Chem. 2001;276:18665–18672. - PubMed
-
- Azimzadeh J., Bornens M. Structure and duplication of the centrosome. J. Cell Sci. 2007;120:2139–2142. - PubMed
-
- Baron A.T., Suman V.J., Nemeth E., Salisbury J.L. The pericentriolar lattice of PtK2 cells exhibits temperature and calcium-modulated behavior. J. Cell Sci. 1994;107:2993–3003. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

