Immunosuppressive effect of bone marrow-derived mesenchymal stem cells in inflammatory microenvironment favours the growth of B16 melanoma cells
- PMID: 21091630
- PMCID: PMC3822946
- DOI: 10.1111/j.1582-4934.2010.01215.x
Immunosuppressive effect of bone marrow-derived mesenchymal stem cells in inflammatory microenvironment favours the growth of B16 melanoma cells
Abstract
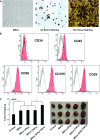
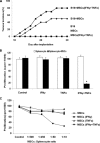
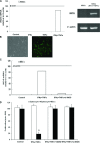
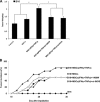
Mesenchymal stem cells (MSCs) are studied for their potential clinical use in regenerative medicine, tissue engineering and tumour therapy. However, the therapeutic application of MSCs in tumour therapy still remains limited unless the immunosuppressive role of MSCs for tumour growth in vivo is better understood. In this study, we investigated the mechanism of MSCs favouring tumour escape from immunologic surveillance in inflammatory microenvironment. We first compared the promotive capacity of bone marrow-derived MSCs on B16 melanoma cells growth in vivo, pre-incubated or not with the inflammatory cytokines interferon (IFN)-γ and tumour necrosis factor (TNF)-α. We showed that the development of B16 melanoma cells is faster when co-injected with MSCs pre-incubated with IFN-γ and TNF-α compared with control groups. Moreover, tumour incidence increases obviously in allogeneic recipients when B16 melanoma cells were co-injected with MSCs pre-incubated with IFN-γ and TNF-α. We then demonstrated that the immunosuppressive function of MSCs was elicited by IFN-γ and TNF-α. These cytokine combinations provoke the expression of inducible nitric oxide synthase (iNOS) by MSCs. The impulsive effect of MSCs treated with inflammatory cytokines on B16 melanoma cells in vivo can be reversed by inhibitor or short interfering RNA of iNOS. Our results suggest that the MSCs in tumour inflammatory microenvironment may be elicited of immunosuppressive function, which will help tumour to escape from the immunity surveillance.
© 2011 The Authors Journal of Cellular and Molecular Medicine © 2011 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures
References
-
- Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. - PubMed
-
- Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568–84. - PubMed
-
- da Silva Meirelles L, Chagastelles PC, et al. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–13. - PubMed
-
- Noel D, Djouad F, Jorgense C. Regenerative medicine through mesenchymal stem cells for bone and cartilage repair. Curr Opin Investig Drugs. 2002;3:1000–4. - PubMed
-
- Sato K, Ozaki K, Oh I, et al. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109:228–34. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

