The glucocorticoid-induced TNF receptor family-related protein (GITR) is critical to the development of acute pancreatitis in mice
- PMID: 21091650
- PMCID: PMC3051390
- DOI: 10.1111/j.1476-5381.2010.01123.x
The glucocorticoid-induced TNF receptor family-related protein (GITR) is critical to the development of acute pancreatitis in mice
Abstract
Background and purpose: Pancreatitis represents a life-threatening inflammatory condition where leucocytes, cytokines and vascular endothelium contribute to the development of the inflammatory disease. The glucocorticoid-induced tumour necrosis factor (TNF) receptor family-related protein (GITR) is a costimulatory molecule for T lymphocytes, modulates innate and adaptive immune system and has been found to participate in a variety of immune responses and inflammatory processes. Our purpose was to verify whether inhibition of GITR triggering results in a better outcome in experimental pancreatitis.
Experimental approach: In male GITR knock-out (GITR(-/-)) and GITR(+/+) mice on Sv129 background, acute pancreatitis was induced after i.p. administration of cerulein. Other experimental groups of GITR(+/+) mice were also treated with different doses of Fc-GITR fusion protein (up to 6.25 µg·mouse⁻¹), given by implanted mini-osmotic pump. Clinical score and pro-inflammatory parameters were evaluated.
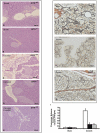
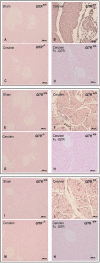
Key results: A less acute pancreatitis was found in GITR(-/-) mice than in GITR(+/+) mice, with marked differences in oedema, neutrophil infiltration, pancreatic dysfunction and injury. Co-treatment of GITR(+/+) mice with cerulein and Fc-GITR fusion protein (6.25 µg·mouse⁻¹) decreased the inflammatory response and tissue injury, compared with treatment with cerulein alone. Inhibition of GITR triggering was found to modulate activation of nuclear factor κB as well as the production of TNF-α, interleukin-1β, inducible nitric oxide synthase, nitrotyrosine, poly-ADP-ribose, intercellular adhesion molecule-1 and P-selectin.
Conclusions and implications: The GITR-GITR ligand system is crucial to the development of acute pancreatitis in mice. Our results also suggest that the Fc-GITR fusion protein could be useful in the treatment of acute pancreatitis.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures







Similar articles
-
Inducible nitric oxide synthase-deficient mice exhibit resistance to the acute pancreatitis induced by cerulein.Shock. 2002 May;17(5):416-22. doi: 10.1097/00024382-200205000-00013. Shock. 2002. PMID: 12022764
-
Proinflammatory role of glucocorticoid-induced TNF receptor-related gene in acute lung inflammation.J Immunol. 2006 Jul 1;177(1):631-41. doi: 10.4049/jimmunol.177.1.631. J Immunol. 2006. PMID: 16785561
-
Glucocorticoid-induced tumor necrosis factor receptor-related (GITR)-Fc fusion protein inhibits GITR triggering and protects from the inflammatory response after spinal cord injury.Mol Pharmacol. 2008 Jun;73(6):1610-21. doi: 10.1124/mol.107.044354. Epub 2008 Mar 5. Mol Pharmacol. 2008. PMID: 18322000
-
GITR: a multifaceted regulator of immunity belonging to the tumor necrosis factor receptor superfamily.Eur J Immunol. 2005 Apr;35(4):1016-22. doi: 10.1002/eji.200425818. Eur J Immunol. 2005. PMID: 15770698 Review.
-
GITR-GITRL system, a novel player in shock and inflammation.ScientificWorldJournal. 2007 May 1;7:533-66. doi: 10.1100/tsw.2007.106. ScientificWorldJournal. 2007. PMID: 17525820 Free PMC article. Review.
Cited by
-
Glucocorticoid-induced tumour necrosis factor receptor family-related protein (GITR) drives atherosclerosis in mice and is associated with an unstable plaque phenotype and cerebrovascular events in humans.Eur Heart J. 2020 Aug 14;41(31):2938-2948. doi: 10.1093/eurheartj/ehaa484. Eur Heart J. 2020. PMID: 32728688 Free PMC article.
-
Pharmacological modulation of GITRL/GITR system: therapeutic perspectives.Br J Pharmacol. 2012 Apr;165(7):2089-99. doi: 10.1111/j.1476-5381.2011.01753.x. Br J Pharmacol. 2012. PMID: 22029729 Free PMC article. Review.
-
GITRL is associated with increased autoantibody production in patients with rheumatoid arthritis.Clin Rheumatol. 2016 Sep;35(9):2195-202. doi: 10.1007/s10067-016-3280-3. Epub 2016 Apr 21. Clin Rheumatol. 2016. PMID: 27098050
-
Modulation of GITR for cancer immunotherapy.Curr Opin Immunol. 2012 Apr;24(2):217-24. doi: 10.1016/j.coi.2011.12.011. Epub 2012 Jan 12. Curr Opin Immunol. 2012. PMID: 22245556 Free PMC article. Review.
-
TNF superfamily in inflammatory disease: translating basic insights.Trends Immunol. 2012 Mar;33(3):144-52. doi: 10.1016/j.it.2011.10.004. Epub 2011 Dec 13. Trends Immunol. 2012. PMID: 22169337 Free PMC article. Review.
References
-
- Bae EM, Kim WJ, Suk K, Kang YM, Park JE, Kim WY, et al. Reverse signaling initiated from GITRL induces NF-kappaB activation through ERK in the inflammatory activation of macrophages. Mol Immunol. 2008;45:523–533. - PubMed
-
- Bhatia M. Novel therapeutic targets for acute pancreatitis and associated multiple organ dysfunction syndrome. Curr Drug Targets Inflamm Allergy. 2002;1:343–351. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

