Metformin inhibits HMGB1 release in LPS-treated RAW 264.7 cells and increases survival rate of endotoxaemic mice
- PMID: 21091653
- PMCID: PMC3057288
- DOI: 10.1111/j.1476-5381.2010.01126.x
Metformin inhibits HMGB1 release in LPS-treated RAW 264.7 cells and increases survival rate of endotoxaemic mice
Abstract
Background and purpose: Recently, metformin, a well-known anti-diabetic drug, has been shown to possess anti-inflammatory activities. This study investigated the effect of metformin on the expression of pro-inflammatory cytokines including high mobility group box 1 (HMGB1) in lipopolysaccharide (LPS)-treated animals and cells.
Experimental approach: We investigated whether metformin inhibits the release of HMGB1 in LPS-treated RAW 264.7 cells and increases survival rate in endotoxaemic mice (lethal endotoxaemia was induced by an i.p. injection of LPS). This was achieved by a range of techniques including Western blotting, enzyme-linked immunosorbent assay, specific pharmacological inhibitors, knock out of α(1) -subunit of AMP-activated protein kinase (AMPK) and recombinant HMGB1.
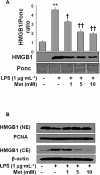
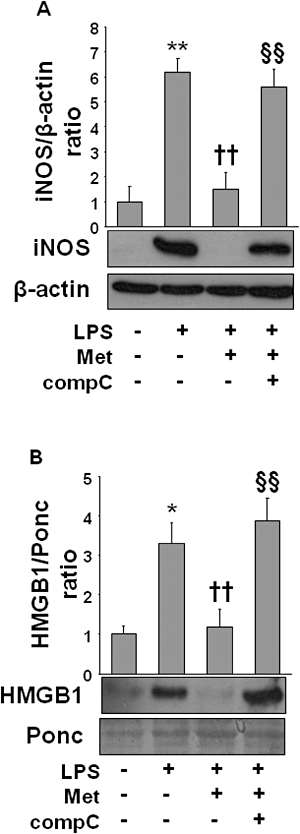
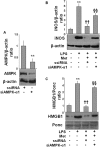
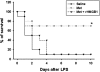
Key results: Both pre- and post-treatment with metformin significantly improved survival of animals during lethal endotoxaemia (survival rate was monitored up to 2 weeks), decreased serum levels of tumour necrosis factor-alpha (TNF-α), interleukin-1β, HMGB1 expression and myeloperoxidase activity in lungs. However, metformin failed to improve survival in endotoxaemic animals that had additionally been treated with recombinant HMGB1. In an in vitro study, metformin dose-dependently inhibited production of pro-inflammatory cytokines and HMGB1 release. Metformin activated AMPK by its phosphorylation. Compound C (pharmacological inhibitor of AMPK) and siAMPKα1 reversed the anti-inflammatory effect of metformin in LPS-treated cells.
Conclusions and implications: Our data indicate that metformin significantly attenuates the pro-inflammatory response induced by LPS both in vivo and in vitro. Metformin improved survival in a mouse model of lethal endotoxaemia by inhibiting HMGB1 release. AMPK activation was implicated as one of the mechanisms contributing to this inhibition of HMGB1 secretion.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures



References
-
- Abdulahad DA, Westra J, Limburg PC, Kallenberg CG, Bijl M. HMGB1 in Systemic Lupus Erythematosus: its role in cutaneous lesions development. Autoimmun Rev. 2010;9:661–665. [Epub ahead of print] - PubMed
-
- Andersson U, Harris HE. The role of HMGB1 in the pathogenesis of rheumatic disease. Biochim Biophys Acta. 2010;1799:141–148. - PubMed
-
- Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334:574–579. - PubMed
-
- Bergheim I, Luyendyk JP, Steele C, Russell GK, Guo L, Roth RA, et al. Metformin prevents endotoxin-induced liver injury after partial hepatectomy. J Pharmacol Exp Ther. 2006;316:1053–1061. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

