Oestrogen-mediated protection of experimental autoimmune encephalomyelitis in the absence of Foxp3+ regulatory T cells implicates compensatory pathways including regulatory B cells
- PMID: 21091909
- PMCID: PMC3044900
- DOI: 10.1111/j.1365-2567.2010.03380.x
Oestrogen-mediated protection of experimental autoimmune encephalomyelitis in the absence of Foxp3+ regulatory T cells implicates compensatory pathways including regulatory B cells
Abstract
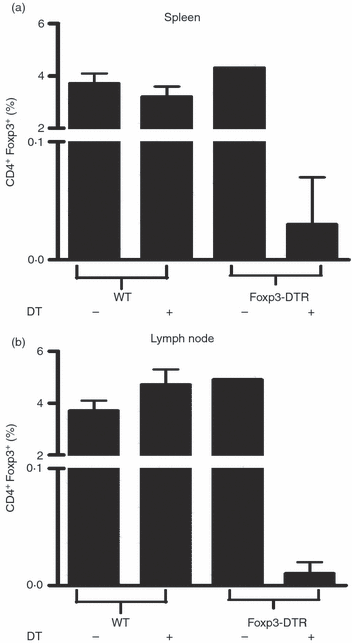
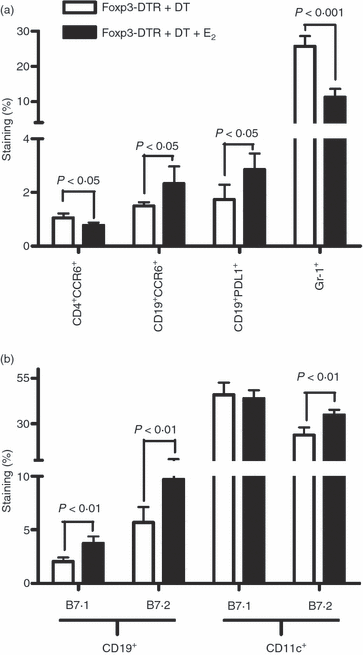
Oestrogen (17β-oestradiol, E₂) is a highly effective treatment for experimental autoimmune encephalomyelitis (EAE) that may potentiate Foxp3+ regulatory T (Treg) cells, which in turn limit the expansion of encephalitogenic T-cell specificities. To determine if Treg cells constitute the major non-redundant protective pathway for E₂, we evaluated E₂ protection of EAE after targeted deletion of Foxp3 expression in Foxp3-DTR mice. Unexpectedly, E₂-treated Foxp3-deficient mice were completely protected against clinical and histological myelin oligodendrocyte glycoprotein (MOG)-35-55 peptide-induced EAE before succumbing to diphtheria toxin-induced mortality. This finding indicated the presence of alternative E₂-dependent EAE-protective pathways that could compensate for the lack of Treg cells. Further investigation revealed that E₂ treatment inhibited proliferation and expression of CCL2 and CXCL2, but enhanced secretion of interleukin-10 (IL-10) and IL-13 by MOG-35-55-specific spleen cells. These changes occurred concomitantly with increased expression of several chemokines and receptors, including CXCL13 and CXCR5, and the negative co-activation molecules, PD-L1 and B7.2, by B cells and dendritic cells. Furthermore, E₂ treatment resulted in higher percentages of spleen and lymph node T cells expressing IL-17, interferon-γ and tumour necrosis factor-α, but with lower expression of CCR6, suggesting sequestration of MOG-35-55 peptide-specific T cells in peripheral immune organs. Taken together, these data suggest that E₂-induced mechanisms that provide protection against EAE in the absence of Foxp3+ Treg cells include induction of regulatory B cells and peripheral sequestration of encephalitogenic T cells.
© 2010 The Authors. Immunology © 2010 Blackwell Publishing Ltd.
Figures






Similar articles
-
Oestrogen treatment of experimental autoimmune encephalomyelitis requires 17β-oestradiol-receptor-positive B cells that up-regulate PD-1 on CD4+ Foxp3+ regulatory T cells.Immunology. 2012 Dec;137(4):282-93. doi: 10.1111/imm.12013. Immunology. 2012. PMID: 23039230 Free PMC article.
-
Involvement of regulatory T cells in the experimental autoimmune encephalomyelitis-preventive effect of dendritic cells expressing myelin oligodendrocyte glycoprotein plus TRAIL.J Immunol. 2007 Jan 15;178(2):918-25. doi: 10.4049/jimmunol.178.2.918. J Immunol. 2007. PMID: 17202353
-
Regulatory B cells (B10 cells) and regulatory T cells have independent roles in controlling experimental autoimmune encephalomyelitis initiation and late-phase immunopathogenesis.J Immunol. 2010 Aug 15;185(4):2240-52. doi: 10.4049/jimmunol.1001307. Epub 2010 Jul 12. J Immunol. 2010. PMID: 20624940 Free PMC article.
-
Discovery and Function of B-Cell IgD Low (BDL) B Cells in Immune Tolerance.J Mol Biol. 2021 Jan 8;433(1):166584. doi: 10.1016/j.jmb.2020.06.023. Epub 2020 Jun 29. J Mol Biol. 2021. PMID: 32615130 Free PMC article. Review.
-
Pathogenic and regulatory roles for B cells in experimental autoimmune encephalomyelitis.Autoimmunity. 2012 Aug;45(5):388-99. doi: 10.3109/08916934.2012.665523. Epub 2012 Apr 19. Autoimmunity. 2012. PMID: 22443691 Free PMC article. Review.
Cited by
-
Differential targeting of IL-2 and T cell receptor signaling pathways selectively expands regulatory T cells while inhibiting conventional T cells.J Autoimmun. 2013 Aug;44:13-20. doi: 10.1016/j.jaut.2013.06.009. Epub 2013 Jul 5. J Autoimmun. 2013. PMID: 23834842 Free PMC article.
-
Neuroprotective effects of estrogens and androgens in CNS inflammation and neurodegeneration.Front Neuroendocrinol. 2012 Jan;33(1):105-15. doi: 10.1016/j.yfrne.2011.12.001. Epub 2011 Dec 24. Front Neuroendocrinol. 2012. PMID: 22209870 Free PMC article. Review.
-
Immunological Processes Driving IgE Sensitisation and Disease Development in Males and Females.Int J Mol Sci. 2018 May 23;19(6):1554. doi: 10.3390/ijms19061554. Int J Mol Sci. 2018. PMID: 29882879 Free PMC article. Review.
-
Sex, Cells, and Asthma.Mayo Clin Proc. 2021 Jul;96(7):1955-1969. doi: 10.1016/j.mayocp.2020.12.007. Mayo Clin Proc. 2021. PMID: 34218868 Free PMC article. Review.
-
Regulatory B Cells in Pregnancy: Lessons from Autoimmunity, Graft Tolerance, and Cancer.Front Immunol. 2017 Feb 17;8:172. doi: 10.3389/fimmu.2017.00172. eCollection 2017. Front Immunol. 2017. PMID: 28261223 Free PMC article. Review.
References
-
- Offner H, Polanczyk M. A potential role for estrogen in experimental autoimmune encephalomyelitis and multiple sclerosis. Ann N Y Acad Sci. 2006;1089:343–72. - PubMed
-
- Jansson L, Holmdahl R. Estrogen-mediated immunosuppression in autoimmune diseases. Inflamm Res. 1998;47:290–301. - PubMed
-
- Zandman-Goddard G, Peeva E, Shoenfeld Y. Gender and autoimmunity. Autoimmun Rev. 2007;6:366–72. - PubMed
-
- Vukusic S, Hutchinson M, Hours M, Moreau T, Cortinovis-Tourniaire P, Adeleine P, Confavreux C. The Pregnancy In Multiple Sclerosis G. Pregnancy and multiple sclerosis (the PRIMS study): clinical predictors of post-partum relapse. Brain. 2004;127(Pt 6):1353–60. - PubMed
-
- Bebo BF, Jr, Fyfe-Johnson A, Adlard K, Beam AG, Vandenbark AA, Offner H. Low-dose estrogen therapy ameliorates experimental autoimmune encephalomyelitis in two different inbred mouse strains. J Immunol. 2001;166:2080–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

