Image cytometric analysis of p53 and mdm-2 expression in primary and recurrent mucoepidermoid carcinoma of parotid gland: immunohistochemical study
- PMID: 21092204
- PMCID: PMC3000838
- DOI: 10.1186/1746-1596-5-72
Image cytometric analysis of p53 and mdm-2 expression in primary and recurrent mucoepidermoid carcinoma of parotid gland: immunohistochemical study
Abstract
Aims and objectives: This study aims to analyze immunocytochemically p53 aberrant expression and mdm-2 expression in primary and recurrent mucoepidermoid carcinoma (MEC) of parotid gland and to ascertain if expression of these markers correlates with tumor behavior, clinical outcome, histological grade and local recurrence.
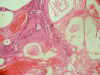
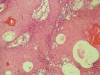
Methods: 20 cases histologically diagnosed as primary MEC with different grades were included in the study. Out of 20 cases, 7 were classified as grade I, 8 as grade II and 5 as grade III. Immunohistochemical staining of these 20 primary cases as well as 6 recurrent cases with anti-p53 and anti-mdm-2 antibodies was carried out. Area fraction of immunopositivity was estimated by image analysis software.
Results: 16/20 primary cases were p53 +ve (80%). The p53 positive cases included 3 cases classified as grade (I), 8 cases as grade (II) and 5 cases as grade (III). All 6 recurrent cases were p53 +ve. On the other hand, 14/20 primary and only 2/6 recurrent cases were mdm-2 +ve. The mdm-2 +ve primary cases included 2 classified as grade (I), 7 as grade (II) and 5 as grade (III). 12 primary MEC showed co-expression of both p53 and mdm-2 of which 2 cases showed local recurrence.
Conclusions: These data suggested that expression of p53 and mdm-2 in primary and recurrent MEC correlates with the high histological grade. P53 aberrant expression is not only considered as an early event in MEC carcinogenesis but also correlates to tumor behavior and local recurrence. Mdm-2 overexpression is correlated to pathogenesis of MEC. However, no strong evidence was found between mdm-2 expression and MEC local recurrence.
Figures






Similar articles
-
Increased expression of MUC-1 has close relation with patient survivor in high-grade salivary gland mucoepidermoid carcinoma.J Oral Pathol Med. 2014 Sep;43(8):579-84. doi: 10.1111/jop.12170. Epub 2014 Feb 14. J Oral Pathol Med. 2014. PMID: 24725122
-
Increased expression of MUC1 predicts poor survival in salivary gland mucoepidermoid carcinoma.J Craniomaxillofac Surg. 2014 Dec;42(8):1891-6. doi: 10.1016/j.jcms.2014.07.008. Epub 2014 Aug 6. J Craniomaxillofac Surg. 2014. PMID: 25187376
-
Elective nodal dissection for cN0 intermediate-grade parotid mucoepidermoid carcinoma: A NCDB study.Am J Otolaryngol. 2024 May-Jun;45(3):104214. doi: 10.1016/j.amjoto.2023.104214. Epub 2024 Jan 6. Am J Otolaryngol. 2024. PMID: 38218029
-
Mucoepidermoid carcinoma involving Warthin tumor. A report of five cases and review of the literature.Am J Clin Pathol. 2000 Oct;114(4):564-70. doi: 10.1309/GUT1-F58A-V4WV-0D8P. Am J Clin Pathol. 2000. PMID: 11026102 Review.
-
Warthin-like Mucoepidermoid Carcinoma of the Parotid Gland: Unusual Morphology and Diagnostic Pitfalls.Anticancer Res. 2019 Jun;39(6):3213-3217. doi: 10.21873/anticanres.13461. Anticancer Res. 2019. PMID: 31177170 Review.
Cited by
-
Whole-Exome Sequencing of Salivary Gland Mucoepidermoid Carcinoma.Clin Cancer Res. 2017 Jan 1;23(1):283-288. doi: 10.1158/1078-0432.CCR-16-0720. Epub 2016 Jun 23. Clin Cancer Res. 2017. PMID: 27340278 Free PMC article.
-
Odontogenic keratocyst: Analysis of recurrence by AgNOR, p53 and MDM2 profiling.J Oral Maxillofac Pathol. 2020 Jan-Apr;24(1):184-185. doi: 10.4103/jomfp.JOMFP_129_19. Epub 2020 May 8. J Oral Maxillofac Pathol. 2020. PMID: 32508473 Free PMC article.
-
Unusual mucoepidermoid carcinoma of the liver misdiagnosed as squamous cell carcinoma by intraoperative histological examination.Diagn Pathol. 2014 Jan 29;9:24. doi: 10.1186/1746-1596-9-24. Diagn Pathol. 2014. PMID: 24475740 Free PMC article.
-
The clinical outcome, pathologic spectrum, and genomic landscape for 454 cases of salivary mucoepidermoid carcinoma.NPJ Precis Oncol. 2024 Oct 22;8(1):238. doi: 10.1038/s41698-024-00735-2. NPJ Precis Oncol. 2024. PMID: 39438706 Free PMC article.
-
Targeting MDM2 for Treatment of Adenoid Cystic Carcinoma.Clin Cancer Res. 2016 Jul 15;22(14):3550-9. doi: 10.1158/1078-0432.CCR-15-1698. Epub 2016 Mar 2. Clin Cancer Res. 2016. PMID: 26936915 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

