Oral tolerance inhibits pulmonary eosinophilia in a cockroach allergen induced model of asthma: a randomized laboratory study
- PMID: 21092270
- PMCID: PMC3016351
- DOI: 10.1186/1465-9921-11-160
Oral tolerance inhibits pulmonary eosinophilia in a cockroach allergen induced model of asthma: a randomized laboratory study
Abstract
Background: Antigen desensitization through oral tolerance is becoming an increasingly attractive treatment option for allergic diseases. However, the mechanism(s) by which tolerization is achieved remain poorly defined. In this study we endeavored to induce oral tolerance to cockroach allergen (CRA: a complex mixture of insect components) in order to ameliorate asthma-like, allergic pulmonary inflammation.
Methods: We compared the pulmonary inflammation of mice which had received four CRA feedings prior to intratracheal allergen sensitization and challenge to mice fed PBS on the same time course. Respiratory parameters were assessed by whole body unrestrained plethysmography and mechanical ventilation with forced oscillation. Bronchoalveolar lavage fluid (BAL) and lung homogenate (LH) were assessed for cytokines and chemokines by ELISA. BAL inflammatory cells were also collected and examined by light microscopy.
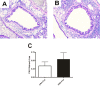
Results: CRA feeding prior to allergen sensitization and challenge led to a significant improvement in respiratory health. Airways hyperreactivity measured indirectly via enhanced pause (Penh) was meaningfully reduced in the CRA-fed mice compared to the PBS fed mice (2.3 ± 0.4 vs 3.9 ± 0.6; p = 0.03). Directly measured airways resistance confirmed this trend when comparing the CRA-fed to the PBS-fed animals (2.97 ± 0.98 vs 4.95 ± 1.41). This effect was not due to reduced traditional inflammatory cell chemotactic factors, Th2 or other cytokines and chemokines. The mechanism of improved respiratory health in the tolerized mice was due to significantly reduced eosinophil numbers in the bronchoalveolar lavage fluid (43300 ± 11445 vs 158786 ± 38908; p = 0.007) and eosinophil specific peroxidase activity in the lung homogenate (0.59 ± 0.13 vs 1.19 ± 0.19; p = 0.017). The decreased eosinophilia was likely the result of increased IL-10 in the lung homogenate of the tolerized mice (6320 ± 354 ng/mL vs 5190 ± 404 ng/mL, p = 0.02).
Conclusion: Our results show that oral tolerization to CRA can improve the respiratory health of experimental mice in a CRA-induced model of asthma-like pulmonary inflammation by reducing pulmonary eosinophilia.
Figures





References
-
- Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic obstructive pulmonary disease surveillance--United States, 1971-2000. MMWR Surveill Summ. 2002;51(6):1–16. - PubMed
-
- National Heart LaBI. Morbidity and Mortality: 2007 Chart Book On Cardiovascular, Lung and Blood Diseases. 2007.
-
- Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, Mitchell H, McNiff-Mortimer K, Lynn H, Ownby D. et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336(19):1356–1363. doi: 10.1056/NEJM199705083361904. - DOI - PubMed
-
- Campbell EM, Kunkel SL, Strieter RM, Lukacs NW. Temporal role of chemokines in a murine model of cockroach allergen-induced airway hyperreactivity and eosinophilia. J Immunol. 1998;161(12):7047–7053. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

