Melanoma-associated Chondroitin Sulfate Proteoglycan (MCSP)-targeted delivery of soluble TRAIL potently inhibits melanoma outgrowth in vitro and in vivo
- PMID: 21092273
- PMCID: PMC3000402
- DOI: 10.1186/1476-4598-9-301
Melanoma-associated Chondroitin Sulfate Proteoglycan (MCSP)-targeted delivery of soluble TRAIL potently inhibits melanoma outgrowth in vitro and in vivo
Abstract
Background: Advanced melanoma is characterized by a pronounced resistance to therapy leading to a limited patient survival of ~6 - 9 months. Here, we report on a novel bifunctional therapeutic fusion protein, designated anti-MCSP:TRAIL, that is comprised of a melanoma-associated chondroitin sulfate proteoglycan (MCSP)-specific antibody fragment (scFv) fused to soluble human TRAIL. MCSP is a well-established target for melanoma immunotherapy and has recently been shown to provide important tumorigenic signals to melanoma cells. TRAIL is a highly promising tumoricidal cytokine with no or minimal toxicity towards normal cells. Anti-MCSP:TRAIL was designed to 1. selectively accrete at the cell surface of MCSP-positive melanoma cells and inhibit MCSP tumorigenic signaling and 2. activate apoptotic TRAIL-signaling.
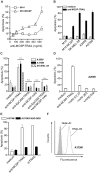
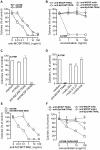
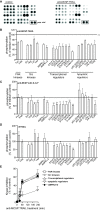
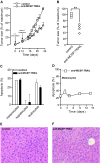
Results: Treatment of a panel of MCSP-positive melanoma cell lines with anti-MCSP:TRAIL induced TRAIL-mediated apoptotic cell death within 16 h. Of note, treatment with anti-MCSP:sTRAIL was also characterized by a rapid dephosphorylation of key proteins, such as FAK, implicated in MCSP-mediated malignant behavior. Importantly, anti-MCSP:TRAIL treatment already inhibited anchorage-independent growth by 50% at low picomolar concentrations, whereas > 100 fold higher concentrations of non-targeted TRAIL failed to reduce colony formation. Daily i.v. treatment with a low dose of anti-MCSP:TRAIL (0.14 mg/kg) resulted in a significant growth retardation of established A375 M xenografts. Anti-MCSP:TRAIL activity was further synergized by co-treatment with rimcazole, a σ-ligand currently in clinical trials for the treatment of various cancers.
Conclusions: Anti-MCSP:TRAIL has promising pre-clinical anti-melanoma activity that appears to result from combined inhibition of tumorigenic MCSP-signaling and concordant activation of TRAIL-apoptotic signaling. Anti-MCSP:TRAIL alone, or in combination with rimcazole, may be of potential value for the treatment of malignant melanoma.
Figures

References
-
- Balch C, Buzaid A, Soong S, Atkins M, Cascinelli N, Coit D, Fleming I, Gershenwald J, Houghton A, Kirkwood J. et al. Final Version of the American Joint Committee on Cancer Staging System for Cutaneous Melanoma. J Clin Oncol. 2001;19:3635–3648. - PubMed
-
- Helmbach H, Sinha P, Schadendorf D. Human melanoma: drug resistance. Recent Results Cancer Res. 2003;161:93–110. - PubMed
-
- Campoli MR, Chang CC, Kageshita T, Wang X, McCarthy JB, Ferrone S. Human high molecular weight-melanoma-associated antigen (HMW-MAA): a melanoma cell surface chondroitin sulfate proteoglycan (MSCP) with biological and clinical significance 1. Crit Rev Immunol. 2004;24:267–296. doi: 10.1615/CritRevImmunol.v24.i4.40. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

