Pharmacokinetic and pharmacodynamic comparison of two "pegylated" interferon alpha-2 formulations in healthy male volunteers: a randomized, crossover, double-blind study
- PMID: 21092287
- PMCID: PMC3001701
- DOI: 10.1186/1471-2210-10-15
Pharmacokinetic and pharmacodynamic comparison of two "pegylated" interferon alpha-2 formulations in healthy male volunteers: a randomized, crossover, double-blind study
Abstract
Background: Interferon (IFN) alpha conjugation to polyethylene glycol (PEG) results in a better pharmacokinetic profile and efficacy. The aim of this study was to compare the pharmacokinetic, pharmacodynamic and safety properties of a new, locally developed, 40-kDa PEG-IFN alpha-2b preparation with a reference, commercially available PEG-IFN alpha-2a in healthy male volunteers.
Methods: A randomized, crossover, double-blind study with a 3-weeks washout period, was done. A single 180 micrograms PEG-IFN alpha-2 dose was administered subcutaneously in both groups. Sixteen apparently healthy male subjects were included. Serum PEG-IFN concentration was measured during 336 hours by an enzyme immunoassay (EIA). Other clinical and laboratory variables were used as pharmacodynamic and safety criteria.
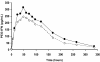
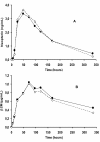
Results: The pharmacokinetic comparison by EIA yielded a high similitude between the formulations. In spite of a high subject variability, the parameters' mean were very close (in all cases p > 0.05): AUC: 53623 vs. 44311 pg.h/mL; Cmax: 333 vs. 271 pg/mL; Tmax: 54 vs. 55 h; half-life (t1/2): 72.4 vs. 64.8 h; terminal elimination rate (lambda): 0.011 vs. 0.014 h(-1); mean residence time (MRT): 135 vs. 123 h for reference and study preparations, respectively. There were no significant differences with respect to the pharmacodynamic variables either: serum neopterin and beta-2 microglobulin levels, stimulation of 2'5' oligoadenylate synthetase expression, and serum IFN antiviral activity. A strong Spearman's rank order correlation (p < 0.01) between the pharmacokinetic and pharmacodynamic concentration-time curves was observed. Both products caused similar leukocyte counts diminution and had similar safety profiles. The most frequent adverse reactions were leukopenia, fever, thrombocytopenia, transaminases increase and asthenia, mostly mild.
Conclusions: Both formulations are fully comparable from the pharmacokinetic, pharmacodynamic, and safety profiles. Efficacy trials can be carried out to confirm clinical similarity.
Figures
Similar articles
-
Pharmacokinetic and pharmacodynamic characterization of a new formulation containing synergistic proportions of interferons alpha-2b and gamma (HeberPAG) in patients with mycosis fungoides: an open-label trial.BMC Pharmacol Toxicol. 2012 Dec 28;13:20. doi: 10.1186/2050-6511-13-20. BMC Pharmacol Toxicol. 2012. PMID: 23272809 Free PMC article. Clinical Trial.
-
Bioequivalence of two recombinant interferon alpha-2b liquid formulations in healthy male volunteers.Drugs R D. 2004;5(5):271-80. doi: 10.2165/00126839-200405050-00003. Drugs R D. 2004. PMID: 15357626 Clinical Trial.
-
A pharmacokinetic and pharmacodynamic comparison of a novel pegylated recombinant consensus interferon-α variant with peginterferon-α-2a in healthy subjects.Br J Clin Pharmacol. 2015 Apr;79(4):650-9. doi: 10.1111/bcp.12528. Br J Clin Pharmacol. 2015. PMID: 25297637 Free PMC article. Clinical Trial.
-
Pegylated interferon with ribavirin therapy for chronic infection with the hepatitis C virus.Expert Opin Pharmacother. 2003 May;4(5):685-91. doi: 10.1517/14656566.4.5.685. Expert Opin Pharmacother. 2003. PMID: 12739994 Review.
-
Development and pharmacokinetics and pharmacodynamics of pegylated interferon alfa-2a (40 kD).Semin Liver Dis. 2004;24 Suppl 2:33-8. doi: 10.1055/s-2004-832926. Semin Liver Dis. 2004. PMID: 15346244 Review.
Cited by
-
Integration of healthy volunteers in early phase clinical trials with immuno-oncological compounds.Front Oncol. 2022 Aug 29;12:954806. doi: 10.3389/fonc.2022.954806. eCollection 2022. Front Oncol. 2022. PMID: 36106110 Free PMC article.
-
Saturable human neopterin response to interferon-α assessed by a pharmacokinetic-pharmacodynamic model.J Transl Med. 2013 Oct 2;11:240. doi: 10.1186/1479-5876-11-240. J Transl Med. 2013. PMID: 24088361 Free PMC article. Clinical Trial.
-
Pharmacokinetic and pharmacodynamic characterization of a new formulation containing synergistic proportions of interferons alpha-2b and gamma (HeberPAG) in patients with mycosis fungoides: an open-label trial.BMC Pharmacol Toxicol. 2012 Dec 28;13:20. doi: 10.1186/2050-6511-13-20. BMC Pharmacol Toxicol. 2012. PMID: 23272809 Free PMC article. Clinical Trial.
-
Molecular characterization of type I IFN-induced cytotoxicity in bladder cancer cells reveals biomarkers of resistance.Mol Ther Oncolytics. 2021 Nov 12;23:547-559. doi: 10.1016/j.omto.2021.11.006. eCollection 2021 Dec 17. Mol Ther Oncolytics. 2021. PMID: 34938855 Free PMC article.
-
Ruxolitinib for the treatment of patients with polycythemia vera.Expert Rev Hematol. 2015 Aug;8(4):391-401. doi: 10.1586/17474086.2015.1045869. Epub 2015 May 17. Expert Rev Hematol. 2015. PMID: 25980454 Free PMC article. Review.
References
-
- Monkarsh SP, Ma Y, Aglione A, Bailon P, Ciolek D, DeBarbieri B, Graves MC, Hollfelder K, Michel H, Palleroni A, Porter JE, Russoman E, Roy S, Pan YC. Positional isomers of monopegylated interferon a-2a: isolation, characterization and biological activity. Analytical Biochem. 1997;247:434–440. doi: 10.1006/abio.1997.2128. - DOI - PubMed
-
- Glue P, Fang JW, Rouzier-Panis R, Raffanel C, Sabo R, Gupta SK, Salfi M, Jacobs S. Pegylated interferon-alpha2b: pharmacokinetics, pharmacodynamics, safety, and preliminary efficacy data. Hepatitis C Intervention Therapy Group. Clin Pharmacol Ther. 2000;68:556–567. doi: 10.1067/mcp.2000.110973. - DOI - PubMed
-
- Ferenci P. PEG IFN alfa-2a (40 KD) (Pegasys) for the treatment of patients with chronic hepatitis C. Int J Clin Pract. 2003;57:610–615. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

