Development and analysis of an in vivo-compatible metabolic network of Mycobacterium tuberculosis
- PMID: 21092312
- PMCID: PMC3225870
- DOI: 10.1186/1752-0509-4-160
Development and analysis of an in vivo-compatible metabolic network of Mycobacterium tuberculosis
Abstract
Background: During infection, Mycobacterium tuberculosis confronts a generally hostile and nutrient-poor in vivo host environment. Existing models and analyses of M. tuberculosis metabolic networks are able to reproduce experimentally measured cellular growth rates and identify genes required for growth in a range of different in vitro media. However, these models, under in vitro conditions, do not provide an adequate description of the metabolic processes required by the pathogen to infect and persist in a host.
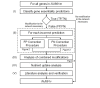
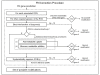
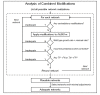
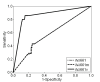
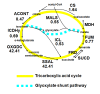
Results: To better account for the metabolic activity of M. tuberculosis in the host environment, we developed a set of procedures to systematically modify an existing in vitro metabolic network by enhancing the agreement between calculated and in vivo-measured gene essentiality data. After our modifications, the new in vivo network contained 663 genes, 838 metabolites, and 1,049 reactions and had a significantly increased sensitivity (0.81) in predicted gene essentiality than the in vitro network (0.31). We verified the modifications generated from the purely computational analysis through a review of the literature and found, for example, that, as the analysis suggested, lipids are used as the main source for carbon metabolism and oxygen must be available for the pathogen under in vivo conditions. Moreover, we used the developed in vivo network to predict the effects of double-gene deletions on M. tuberculosis growth in the host environment, explore metabolic adaptations to life in an acidic environment, highlight the importance of different enzymes in the tricarboxylic acid-cycle under different limiting nutrient conditions, investigate the effects of inhibiting multiple reactions, and look at the importance of both aerobic and anaerobic cellular respiration during infection.
Conclusions: The network modifications we implemented suggest a distinctive set of metabolic conditions and requirements faced by M. tuberculosis during host infection compared with in vitro growth. Likewise, the double-gene deletion calculations highlight the importance of specific metabolic pathways used by the pathogen in the host environment. The newly constructed network provides a quantitative model to study the metabolism and associated drug targets of M. tuberculosis under in vivo conditions.
Figures


References
-
- WHO. WHO Report 2008: Global tuberculosis control - surveillance, planning, financing. 2008.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

