Bovipain-2, the falcipain-2 ortholog, is expressed in intraerythrocytic stages of the tick-transmitted hemoparasite Babesia bovis
- PMID: 21092313
- PMCID: PMC3003645
- DOI: 10.1186/1756-3305-3-113
Bovipain-2, the falcipain-2 ortholog, is expressed in intraerythrocytic stages of the tick-transmitted hemoparasite Babesia bovis
Abstract
Background: Cysteine proteases have been shown to be highly relevant for Apicomplexan parasites. In the case of Babesia bovis, a tick-transmitted hemoparasite of cattle, inhibitors of these enzymes were shown to hamper intraerythrocytic replication of the parasite, underscoring their importance for survival.
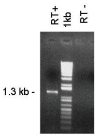
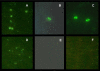
Results: Four papain-like cysteine proteases were found to be encoded by the B. bovis genome using the MEROPS database. One of them, the ortholog of Plasmodium falciparum falcipain-2, here named bovipain-2, was further characterized. Bovipain-2 is encoded in B. bovis chromosome 4 by an ORF of 1.3 kb, has a predicted molecular weight of 42 kDa, and is hydrophilic with the exception of a transmembrane region. It has orthologs in several other apicomplexans, and its predicted amino acid sequence shows a high degree of conservation among several B. bovis isolates from North and South America. Synteny studies demonstrated that the bovipain-2 gene has expanded in the genomes of two related piroplasmids, Theileria parva and T. annulata, into families of 6 and 7 clustered genes respectively. The bovipain-2 gene is transcribed in in vitro cultured intra-erythrocyte forms of a virulent and an attenuated B. bovis strain from Argentina, and has no introns, as shown by RT-PCR followed by sequencing. Antibodies against a recombinant form of bovipain-2 recognized two parasite protein bands of 34 and 26 kDa, which coincide with the predicted sizes of the pro-peptidase and mature peptidase, respectively. Immunofluorescence studies showed an intracellular localization of bovipain-2 in the middle-rear region of in vitro cultured merozoites, as well as diffused in the cytoplasm of infected erythrocytes. Anti-bovipain-2 antibodies also reacted with B. bigemina-infected erythrocytes giving a similar pattern, which suggests cross-reactivity among these species. Antibodies in sera of two out of six B. bovis-experimentally infected bovines tested, reacted specifically with recombinant bovipain-2 in immunoblots, thus demonstrating expression and immunogenicity during bovine-infecting stages.
Conclusions: Overall, we present the characterization of bovipain-2 and demonstrate its in vitro and in vivo expression in virulent and attenuated strains. Given the involvement of apicomplexan cysteine proteases in essential parasite functions, bovipain-2 constitutes a new vaccine candidate and potential drug target for bovine babesiosis.
Figures



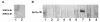
References
-
- Florin-Christensen M, Schnittger L, Dominguez M, Mesplet M, Rodriguez A, Ferreri L, Asenzo G, Wilkowsky S, Farber M, Echaide I, Suarez C. Search for Babesia bovis vaccine candidates. Parassitologia. 2007;49(Suppl 1):9–12. - PubMed
-
- Brayton KA, Lau AO, Herndon DR, Hannick L, Kappmeyer LS, Berens SJ, Bidwell SL, Brown WC, Crabtree J, Fadrosh D, Feldblum T, Forberger HA, Haas BJ, Howell JM, Khouri H, Koo H, Mann DJ, Norimine J, Paulsen IT, Radune D, Ren Q, Smith RK Jr, Suarez CE, White O, Wortman JR, Knowles DP Jr, McElwain TF, Nene VM. Genome sequence of Babesia bovis and comparative analysis of apicomplexan hemoprotozoa. PLoS Pathog. 2007;3:1401–13. doi: 10.1371/journal.ppat.0030148. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

