Suppression of nitric oxide production from nasal fibroblasts by metabolized clarithromycin in vitro
- PMID: 21092318
- PMCID: PMC3003651
- DOI: 10.1186/1476-9255-7-56
Suppression of nitric oxide production from nasal fibroblasts by metabolized clarithromycin in vitro
Abstract
Background: Low-dose and long-term administration of 14-membered macrolide antibiotics, so called macrolide therapy, has been reported to favorably modify the clinical conditions of chronic airway diseases. Since there is growing evidence that macrolide antibiotic-resistant bacteria's spreaders in the populations received macrolide therapy, it is strongly desired to develop macrolide antibiotics, which showed only anti-inflammatory action. The present study was designed to examine the influence of clarithromycin (CAM) and its metabolized materials, M-1, M-4 and M-5, on free radical generation from nasal polyp fibroblasts (NPFs) through the choice of nitric oxide (NO), which is one of important effector molecule in the development of airway inflammatory disease in vitro.
Methods: NPFs (5 × 105 cells/ml) were stimulated with 1.0 μg/ml lipopolysaccharide (LPS) in the presence of agents for 24 hours. NO levels in culture supernatants were examined by the Griess method. We also examined the influence of agents on the phosphorylation of MAPKs, NF-κB activation, iNOS mRNA expression and iNOS production in NPFs cultured for 2, 4, 8, and 12 hours, respectively.
Results: The addition of CAM (> 0.4 μg/ml) and M-4 (> 0.04 μg/ml) could suppress NO production from NPFs after LPS stimulation through the suppression of iNOS mRNA expression and NF-κB activation. CAM and M-4 also suppressed phosphorylation of MAPKs, ERK and p38 MAPK, but not JNK, which are increased LPS stimulation. On the other hand, M-1 and M-5 could not inhibit the NO generation, even when 0.1 μg/ml of the agent was added to cell cultures.
Conclusion: The present results may suggest that M-4 will be a good candidate for the agent in the treatment of chronic airway inflammatory diseases, since M-4 did not have antimicribiological effects on gram positive and negative bacteria.
Figures




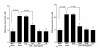
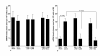



Similar articles
-
Enhancement of thioredoxin production from nasal epithelial cells by the macrolide antibiotic, clarithromycin in vitro.In Vivo. 2013 May-Jun;27(3):351-6. In Vivo. 2013. PMID: 23606690
-
Suppressive activity of macrolide antibiotics on nitric oxide production from nasal polyp fibroblasts in vitro.Acta Otolaryngol. 2003 Dec;123(9):1064-9. doi: 10.1080/00016480310002519. Acta Otolaryngol. 2003. PMID: 14710909
-
Suppressive activity of macrolide antibiotics on nitric oxide production by lipopolysaccharide stimulation in mice.Mediators Inflamm. 2003 Aug;12(4):195-202. doi: 10.1080/09629350310001599620. Mediators Inflamm. 2003. PMID: 14514469 Free PMC article.
-
Long-term treatment of clarithromycin at a low concentration improves hydrogen peroxide-induced oxidant/antioxidant imbalance in human small airway epithelial cells by increasing Nrf2 mRNA expression.BMC Pharmacol Toxicol. 2017 Feb 25;18(1):15. doi: 10.1186/s40360-017-0119-8. BMC Pharmacol Toxicol. 2017. PMID: 28235416 Free PMC article.
-
Xanthii fructus inhibits inflammatory responses in LPS-stimulated RAW 264.7 macrophages through suppressing NF-κB and JNK/p38 MAPK.J Ethnopharmacol. 2015 Dec 24;176:394-401. doi: 10.1016/j.jep.2015.11.020. Epub 2015 Nov 10. J Ethnopharmacol. 2015. PMID: 26560439
Cited by
-
Efficacy of clarithromycin as a protective agent in the methotrexate-induced pulmonary fibrosis model.Kardiochir Torakochirurgia Pol. 2018 Dec;15(4):209-212. doi: 10.5114/kitp.2018.80915. Epub 2018 Dec 31. Kardiochir Torakochirurgia Pol. 2018. PMID: 30647742 Free PMC article.
-
Suppressive Effect of Quercetin on Nitric Oxide Production from Nasal Epithelial Cells In Vitro.Evid Based Complement Alternat Med. 2018 Jul 5;2018:6097625. doi: 10.1155/2018/6097625. eCollection 2018. Evid Based Complement Alternat Med. 2018. PMID: 30069224 Free PMC article.
-
Inhibitory effect of baicalin on iNOS and NO expression in intestinal mucosa of rats with acute endotoxemia.PLoS One. 2013 Dec 2;8(12):e80997. doi: 10.1371/journal.pone.0080997. eCollection 2013. PLoS One. 2013. PMID: 24312512 Free PMC article.
References
-
- Keichou N, Kudoh S. Diffuse panbronchiolitis: role of macrolides in therapy. Am J Respir Med. 2002;1:119–131. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

