CYP17 inhibitors for prostate cancer therapy
- PMID: 21092758
- PMCID: PMC3047603
- DOI: 10.1016/j.jsbmb.2010.11.005
CYP17 inhibitors for prostate cancer therapy
Abstract
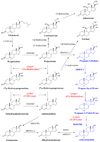
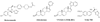
Prostate cancer (PC) is now the second most prevalent cause of death in men in the USA and Europe. At present, the major treatment options include surgical or medical castration. These strategies cause ablation of the production of testosterone (T), dihydrotestosterone (DHT) and related androgens by the testes. However, because these procedures do not affect adrenal, prostate and other tissues' androgen production, they are often combined with androgen receptor antagonists to block their action. Indeed, recent studies have unequivocally established that in castration-resistant prostate cancer (CRPC) many androgen-regulated genes become re-expressed and tissue androgen levels increase despite low serum levels. Clearly, inhibition of the key enzyme which catalyzes the biosynthesis of androgens from pregnane precursors, 17α-hydroxy/17,20-lyase (hereafter referred to as CYP17) could prevent androgen production from all sources. Thus, total ablation of androgen production by potent CYP17 inhibitors may provide effective treatment of prostate cancer patients. This review highlights the role of androgen biosynthesis in the progression of prostate cancer and the impact of CYP17 inhibitors, such as ketoconazole, abiraterone acetate, VN/124-1 (TOK-001) and TAK-700 in the clinic and in clinical development. Article from the special issue on Targeted Inhibitors.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Inhibitors of 17alpha-hydroxylase/17,20-lyase (CYP17): potential agents for the treatment of prostate cancer.Curr Pharm Des. 1999 Mar;5(3):163-80. Curr Pharm Des. 1999. PMID: 10066888 Review.
-
Targeting CYP17: established and novel approaches in prostate cancer.Curr Opin Pharmacol. 2008 Aug;8(4):449-57. doi: 10.1016/j.coph.2008.06.004. Epub 2008 Jul 28. Curr Opin Pharmacol. 2008. PMID: 18619560 Review.
-
Abiraterone acetate: oral androgen biosynthesis inhibitor for treatment of castration-resistant prostate cancer.Drug Des Devel Ther. 2012;6:13-8. doi: 10.2147/DDDT.S15850. Epub 2012 Jan 16. Drug Des Devel Ther. 2012. PMID: 22291466 Free PMC article. Review.
-
The hunt for a selective 17,20 lyase inhibitor; learning lessons from nature.J Steroid Biochem Mol Biol. 2016 Oct;163:136-46. doi: 10.1016/j.jsbmb.2016.04.021. Epub 2016 May 3. J Steroid Biochem Mol Biol. 2016. PMID: 27154414 Free PMC article. Review.
-
Androgen synthesis inhibitors in the treatment of castration-resistant prostate cancer.Asian J Androl. 2014 May-Jun;16(3):387-400. doi: 10.4103/1008-682X.129133. Asian J Androl. 2014. PMID: 24759590 Free PMC article. Review.
Cited by
-
Identification of Novel Steroidal Androgen Receptor Degrading Agents Inspired by Galeterone 3β-Imidazole Carbamate.ACS Med Chem Lett. 2016 May 23;7(7):708-13. doi: 10.1021/acsmedchemlett.6b00137. eCollection 2016 Jul 14. ACS Med Chem Lett. 2016. PMID: 27437082 Free PMC article.
-
Etiology and management of hypertension in patients with cancer.Cardiooncology. 2021 Apr 6;7(1):14. doi: 10.1186/s40959-021-00101-2. Cardiooncology. 2021. PMID: 33823943 Free PMC article. Review.
-
Systematic structure modifications of multitarget prostate cancer drug candidate galeterone to produce novel androgen receptor down-regulating agents as an approach to treatment of advanced prostate cancer.J Med Chem. 2013 Jun 27;56(12):4880-98. doi: 10.1021/jm400048v. Epub 2013 Jun 7. J Med Chem. 2013. PMID: 23713567 Free PMC article.
-
Cardiovascular Effects of Androgen Deprivation Therapy in Prostate Cancer: Contemporary Meta-Analyses.Arterioscler Thromb Vasc Biol. 2020 Mar;40(3):e55-e64. doi: 10.1161/ATVBAHA.119.313046. Epub 2020 Jan 23. Arterioscler Thromb Vasc Biol. 2020. PMID: 31969015 Free PMC article. Review.
-
Effects of abiraterone acetate on chronic kidney disease in 2 patients with metastatic castration-resistant prostate cancer.Medicine (Baltimore). 2014 Dec;93(27):e163. doi: 10.1097/MD.0000000000000163. Medicine (Baltimore). 2014. PMID: 25501058 Free PMC article.
References
-
- American Cancer Society Facts and Figures 2009. 2009.
-
- Denis LJ, Griffiths K. Endocrine treatment in prostate cancer. Seminars in surgical oncology. 2000;18(1):52–74. - PubMed
-
- Mohler JL, Gregory CW, Ford OH, 3rd, Kim D, Weaver CM, Petrusz P, Wilson EM, French FS. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10(2):440–448. - PubMed
-
- Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev. 1997;18(3):306–360. - PubMed
-
- Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1(1):34–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

