NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration
- PMID: 21092857
- PMCID: PMC2989827
- DOI: 10.1016/j.neuron.2010.09.009
NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration
Abstract
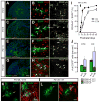
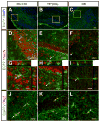
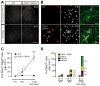
The mammalian CNS contains a ubiquitous population of glial progenitors known as NG2+ cells that have the ability to develop into oligodendrocytes and undergo dramatic changes in response to injury and demyelination. Although it has been reported that NG2+ cells are multipotent, their fate in health and disease remains controversial. Here, we generated PDGFαR-CreER transgenic mice and followed their fate in vivo in the developing and adult CNS. These studies revealed that NG2+ cells in the postnatal CNS generate myelinating oligodendrocytes, but not astrocytes or neurons. In regions of neurodegeneration in the spinal cord of ALS mice, NG2+ cells exhibited enhanced proliferation and accelerated differentiation into oligodendrocytes but remained committed to the oligodendrocyte lineage. These results indicate that NG2+ cells in the normal CNS are oligodendrocyte precursors with restricted lineage potential and that cell loss and gliosis are not sufficient to alter the lineage potential of these progenitors.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

