A bioactive self-assembled membrane to promote angiogenesis
- PMID: 21093042
- PMCID: PMC3150553
- DOI: 10.1016/j.biomaterials.2010.10.048
A bioactive self-assembled membrane to promote angiogenesis
Abstract
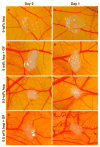
We report here on a bioactive hierarchically structured membrane formed by self-assembly. The membrane is formed with hyaluronic acid and peptide amphiphiles with binding affinity for heparin, and its hierarchical structure contains both an amorphous zone and a layer of fibrils oriented perpendicular to the membrane plane. The design of bioactivity is based on the potential ability to bind and slowly release heparin-binding growth factors. Human mesenchymal stem cells (hMSCs) seeded on these membranes attached and remained viable. Basic fibroblast growth factor (FGF2) and vascular endothelial growth factor (VEGF) were incorporated within the membrane structure prior to self-assembly and released into media over a prolonged period of time (14 days). Using the chicken chorioallantoic membrane (CAM) assay, we also found that these membranes induced a significant and rapid enhancement of angiogenesis relative to controls.
2010 Elsevier Ltd. All rights reserved.
Figures






References
-
- Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428(6982):487–492. - PubMed
-
- Rinaudo M. Main properties and current applications of some polysaccharides as biomaterials. Polym Int. 2008;57(3):397–430.
-
- Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, Stupp SI. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303(5662):1352–1355. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

