Hedgehog signaling in the liver
- PMID: 21093090
- PMCID: PMC3053023
- DOI: 10.1016/j.jhep.2010.10.003
Hedgehog signaling in the liver
Abstract
Reactivation of Hedgehog (Hh), a morphogenic signaling pathway that controls progenitor cell fate and tissue construction during embryogenesis occurs during many types of liver injury in adult. The net effects of activating the Hedgehog pathway include expansion of liver progenitor populations to promote liver regeneration, but also hepatic accumulation of inflammatory cells, liver fibrogenesis, and vascular remodeling. All of these latter responses are known to be involved in the pathogenesis of cirrhosis. In addition, Hh signaling may play a role in primary liver cancers, such as cholangiocarcinoma and hepatocellular carcinoma. Study of Hedgehog signaling in liver cells is in its infancy. Additional research in this area is justified given growing experimental and clinical data supporting a role for the pathway in regulating outcomes of liver injury.
Copyright © 2010 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Figures
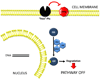



References
-
- Adolphe C, Narang M, Ellis T, Wicking C, Kaur P, Wainwright B. An in vivo comparative study of sonic, desert and Indian hedgehog reveals that hedgehog pathway activity regulates epidermal stem cell homeostasis. Development. 2004;131(20):5009–5019. - PubMed
-
- Alinger B, Kiesslich T, Datz C, Aberger F, Strasser F, Berr F, et al. Hedgehog signaling is involved in differentiation of normal colonic tissue rather than in tumor proliferation. Virchows Arch. 2009;454(4):369–379. - PubMed
-
- Alvaro D, Mancino MG. New insights on the molecular and cell biology of human cholangiopathies. Mol Aspects Med. 2008;29(1–2):50–57. - PubMed
-
- Baum B, Settleman J, Quinlan MP. Transitions between epithelial and mesenchymal states in development and disease. Semin Cell Dev Biol. 2008;19(3):294–308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

