Endothelial heparan sulfate controls chemokine presentation in recruitment of lymphocytes and dendritic cells to lymph nodes
- PMID: 21093315
- PMCID: PMC2996097
- DOI: 10.1016/j.immuni.2010.10.018
Endothelial heparan sulfate controls chemokine presentation in recruitment of lymphocytes and dendritic cells to lymph nodes
Erratum in
- Immunity. 2011 May 27;34(5):820. Petryanik, Bronislawa [corrected to Petryniak, Bronislawa]
Abstract
Heparan sulfate can bind several adhesion molecules involved in lymphocyte trafficking. However, the in vivo function of endothelial heparan sulfate in lymphocyte homing and stimulation of the immune response has not been elucidated. Here, we generated mutant mice deficient in the enzyme Ext1, which is required for heparan sulfate synthesis, in a Tek-dependent and inducible manner. Chemokine presentation was diminished in the mutant mice, causing the lack of appropriate integrin-mediated adhesion, and resulted in a marked decrease in lymphocyte sticking to high endothelial venules and in recruitment of resident dendritic cells through lymphatic vessels to the lymph nodes. As a consequence, mutant mice displayed a severe impairment in lymphocyte homing and a compromised contact hypersensitivity response. By contrast, lymphocyte rolling was increased because of loss of electrostatic repulsion by heparan sulfate. These results demonstrate critical roles of endothelial heparan sulfate in immune surveillance and immune response generation.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures



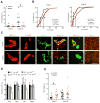


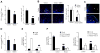
Comment in
-
Trapped versus soluble chemokines: functions in leukocyte adhesion and motility.Immunity. 2010 Nov 24;33(5):654-6. doi: 10.1016/j.immuni.2010.11.012. Immunity. 2010. PMID: 21094462
References
-
- Arbones ML, Ord DC, Ley K, Ratech H, Maynard-Curry C, Otten G, Capon DJ, Tedder TF. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity. 1994;1:247–260. - PubMed
-
- Bishop JR, Schuksz M, Esko JD. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature. 2007;446:1030–1037. - PubMed
-
- Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

