MicroRNAs prevent the generation of autoreactive antibodies
- PMID: 21093320
- PMCID: PMC3687137
- DOI: 10.1016/j.immuni.2010.11.010
MicroRNAs prevent the generation of autoreactive antibodies
Abstract
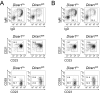
MicroRNAs have been shown to be critical for a number of aspects of immune system regulation and function. Here, we have examined the role of microRNAs in terminal B cell differentiation by analyzing Cd19-Cre(ki/+) Dicer1(fl/fl) mice. We found that in the absence of Dicer, the transitional and marginal zone (MZ) B cell compartments were overrepresented and follicular (FO) B cell generation was impaired. microRNA analysis revealed that miR185, a microRNA overexpressed in FO cells, dampened B cell receptor (BCR) signaling through Bruton tyrosine kinase downregulation. Dicer-deficient B cells had a skewed BCR repertoire with hallmarks of autoreactivity, which correlated with high titers of autoreactive antibodies in serum and autoimmune features in females. Together, our results reveal a crucial role for microRNAs in late B cell differentiation and in the establishment of B cell tolerance.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
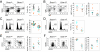


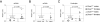

References
-
- Bassing CH, Swat W, Alt FW. The mechanism and regulation of chromosomal V(D)J recombination. Cell. 2002;109(Suppl):S45–55. - PubMed
-
- Carsetti R, Rosado MM, Wardmann H. Peripheral development of B cells in mouse and man. Immunol Rev. 2004;197:179–191. - PubMed
-
- Crouzier R, Martin T, Pasquali JL. Heavy chain variable region, light chain variable region, and heavy chain CDR3 influences on the mono- and polyreactivity and on the affinity of human monoclonal rheumatoid factors. J Immunol. 1995;154:4526–4535. - PubMed
-
- Dammers PM, Visser A, Popa ER, Nieuwenhuis P, Kroese FG. Most marginal zone B cells in rat express germline encoded Ig VH genes and are ligand selected. J Immunol. 2000;165:6156–6169. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

