Betaine-homocysteine methyltransferase: human liver genotype-phenotype correlation
- PMID: 21093336
- PMCID: PMC3053054
- DOI: 10.1016/j.ymgme.2010.10.010
Betaine-homocysteine methyltransferase: human liver genotype-phenotype correlation
Abstract
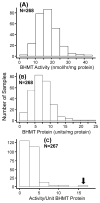
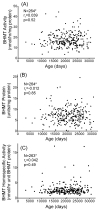
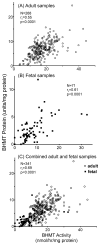
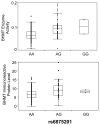
Betaine-homocysteine methyltransferase (BHMT) catalyzes the remethylation of homocysteine. BHMT is highly expressed in the human liver. In the liver, BHMT catalyzes up to 50% of homocysteine metabolism. Understanding the relationship between BHMT genetic polymorphisms and function might increase our understanding of the role of this reaction in homocysteine remethylation and in S-adenosylmethionine-dependent methylation. To help achieve those goals, we measured levels of BHMT enzyme activity and immunoreactive protein in 268 human hepatic surgical biopsy samples from adult subjects as well as 73 fetal hepatic tissue samples obtained at different gestational ages. BHMT protein levels were correlated significantly (p<0.001) with levels of enzyme activity in both fetal and adult tissues, but both were decreased in fetal tissue when compared with levels in the adult hepatic biopsies. To determine possible genotype-phenotype correlations, 12 tag SNPs for BHMT and the closely related BHMT2 gene were selected from SNPs observed during our own gene resequencing studies as well as from HapMap. These SNPs data were used to genotype DNA from the adult hepatic surgical biopsy samples, and genotype-phenotype association analysis was performed. Three SNPs (rs41272270, rs16876512, and rs6875201), located 28kb upstream, in the 5'-UTR and in intron 1 of BHMT, respectively, were significantly correlated with both BHMT activity (p=3.41E-8, 2.55E-9 and 2.46E-10, respectively) and protein levels (p=5.78E-5, 1.08E-5 and 6.92E-6, respectively). We also imputed 230 additional SNPs across the BHMT and BHMT2 genes, identifying an additional imputed SNP, rs7700790, that was also highly associated with hepatic BHMT enzyme activity and protein. However, none of the 3 genotyped or one imputed SNPs displayed a "shift" during electrophoretic mobility shift assays. These observations may help us to understand individual variation in the regulation of BHMT in the human liver and its possible relationship to variation in methylation.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
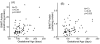

Similar articles
-
Human betaine-homocysteine methyltransferase (BHMT) and BHMT2: common gene sequence variation and functional characterization.Mol Genet Metab. 2008 Jul;94(3):326-35. doi: 10.1016/j.ymgme.2008.03.013. Epub 2008 May 23. Mol Genet Metab. 2008. PMID: 18457970 Free PMC article.
-
Splicing variants of the porcine betaine-homocysteine S-methyltransferase gene: implications for mammalian metabolism.Gene. 2013 Oct 25;529(2):228-37. doi: 10.1016/j.gene.2013.07.103. Epub 2013 Aug 13. Gene. 2013. PMID: 23948084 Free PMC article.
-
A splicing variant leads to complete loss of function of betaine-homocysteine methyltransferase (BHMT) gene in hepatocellular carcinoma.Int J Biochem Cell Biol. 2012 Feb;44(2):385-92. doi: 10.1016/j.biocel.2011.11.014. Epub 2011 Nov 26. Int J Biochem Cell Biol. 2012. PMID: 22138536
-
Betaine homocysteine methyltransferase (BHMT)-dependent remethylation pathway in human healthy and tumoral liver.Clin Chem Lab Med. 2013 Mar 1;51(3):617-21. doi: 10.1515/cclm-2012-0689. Clin Chem Lab Med. 2013. PMID: 23449526 Review.
-
Betaine homocysteine S-methyltransferase: just a regulator of homocysteine metabolism?Cell Mol Life Sci. 2006 Dec;63(23):2792-803. doi: 10.1007/s00018-006-6249-6. Cell Mol Life Sci. 2006. PMID: 17086380 Free PMC article. Review.
Cited by
-
Torularhodin Alleviates Hepatic Dyslipidemia and Inflammations in High-Fat Diet-Induced Obese Mice via PPARα Signaling Pathway.Molecules. 2022 Sep 27;27(19):6398. doi: 10.3390/molecules27196398. Molecules. 2022. PMID: 36234935 Free PMC article.
-
Methionine as a regulator of bone remodeling with fasting.JCI Insight. 2024 May 21;9(12):e177997. doi: 10.1172/jci.insight.177997. JCI Insight. 2024. PMID: 38780544 Free PMC article.
-
Molecular variation and horizontal gene transfer of the homocysteine methyltransferase gene mmuM and its distribution in clinical pathogens.Int J Biol Sci. 2015 Jan 1;11(1):11-21. doi: 10.7150/ijbs.10320. eCollection 2015. Int J Biol Sci. 2015. PMID: 25552925 Free PMC article.
-
The Pediatric Methionine Requirement Should Incorporate Remethylation Potential and Transmethylation Demands.Adv Nutr. 2016 May 16;7(3):523-34. doi: 10.3945/an.115.010843. Print 2016 May. Adv Nutr. 2016. PMID: 27184279 Free PMC article. Review.
-
Genetic variants associated with glycine metabolism and their role in insulin sensitivity and type 2 diabetes.Diabetes. 2013 Jun;62(6):2141-50. doi: 10.2337/db12-0876. Epub 2013 Feb 1. Diabetes. 2013. PMID: 23378610 Free PMC article.
References
-
- Garrow TA. Purification, kinetic properties, and cDNA cloning of mammalian betaine-homocysteine methyltransferase. J Biol Chem. 1996;271:22831–22838. - PubMed
-
- Millian NS, Garrow TA. Human betaine-homocysteine methyltransferase is a zinc metalloenzyme. Arch Biochem Biophys. 1998;356:93–98. - PubMed
-
- Finkelstein JD. Metabolic regulatory properties of S-adenosylmethionine and S-adenosylhomocysteine. Clin Chem Lab Med. 2007;45:1694–1699. - PubMed
-
- Park EI, Garrow TA. Interaction between dietary methionine and methyl donor intake on rat liver betaine-homocysteine methyltransferase gene expression and organization of the human gene. J Biol Chem. 1999;274:7816–7824. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

