Neuronal control of swimming behavior: comparison of vertebrate and invertebrate model systems
- PMID: 21093529
- PMCID: PMC3034781
- DOI: 10.1016/j.pneurobio.2010.11.001
Neuronal control of swimming behavior: comparison of vertebrate and invertebrate model systems
Abstract
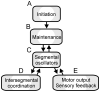
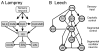
Swimming movements in the leech and lamprey are highly analogous, and lack homology. Thus, similarities in mechanisms must arise from convergent evolution rather than from common ancestry. Despite over 40 years of parallel investigations into this annelid and primitive vertebrate, a close comparison of the approaches and results of this research is lacking. The present review evaluates the neural mechanisms underlying swimming in these two animals and describes the many similarities that provide intriguing examples of convergent evolution. Specifically, we discuss swim initiation, maintenance and termination, isolated nervous system preparations, neural-circuitry, central oscillators, intersegmental coupling, phase lags, cycle periods and sensory feedback. Comparative studies between species highlight mechanisms that optimize behavior and allow us a broader understanding of nervous system function.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures



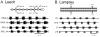
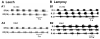
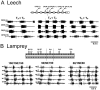
 ) indicate monosynaptic connections; arrows indicate excitatory polysynaptic pathways that are not identified. RLR – rostrolateral rhombencephalon ; MLR – mesencepalic locomotor region; DLM – dorsolateral mesencepalon ; VMD – ventromedial diencepalon; DLR – diencepalic locomotor region ; RS – reticulospinal.
) indicate monosynaptic connections; arrows indicate excitatory polysynaptic pathways that are not identified. RLR – rostrolateral rhombencephalon ; MLR – mesencepalic locomotor region; DLM – dorsolateral mesencepalon ; VMD – ventromedial diencepalon; DLR – diencepalic locomotor region ; RS – reticulospinal.

 ) denote inhibitory synapses; those terminating with a Y (
) denote inhibitory synapses; those terminating with a Y ( ) are excitatory; diode symbols denote rectifying electrical junctions.
) are excitatory; diode symbols denote rectifying electrical junctions.




References
-
- Archambault PS, Deliagina TG, Orlovsky GN. Non-undulatory locomotion in the lamprey. Neuroreport. 2001;12:1803–1807. - PubMed
-
- Bowtell G, Williams TL. Anguilliform body dynamics: modelling the interaction between muscle activation and body curvature. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1991;334:385–390. - PubMed
-
- Boyd MR, McClellan AD. Changes in locomotor activity parameters with variations in cycle time in larval lamprey. J. Exp. Biol. 2002;205:3707–3716. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

