Redox regulation of the mitochondrial K(ATP) channel in cardioprotection
- PMID: 21094666
- PMCID: PMC3109179
- DOI: 10.1016/j.bbamcr.2010.11.005
Redox regulation of the mitochondrial K(ATP) channel in cardioprotection
Abstract
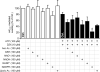
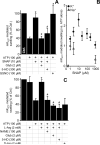
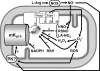
The mitochondrial ATP-sensitive potassium channel (mK(ATP)) is important in the protective mechanism of ischemic preconditioning (IPC). The channel is reportedly sensitive to reactive oxygen and nitrogen species, and the aim of this study was to compare such species in parallel, to build a more comprehensive picture of mK(ATP) regulation. mK(ATP) activity was measured by both osmotic swelling and Tl(+) flux assays, in isolated rat heart mitochondria. An isolated adult rat cardiomyocyte model of ischemia-reperfusion (IR) injury was also used to determine the role of mK(ATP) in cardioprotection by nitroxyl. Key findings were as follows: (i) mK(ATP) was activated by O(2)(-) and H(2)O(2) but not other peroxides. (ii) mK(ATP) was inhibited by NADPH. (iii) mK(ATP) was activated by S-nitrosothiols, nitroxyl, and nitrolinoleate. The latter two species also inhibited mitochondrial complex II. (iv) Nitroxyl protected cardiomyocytes against IR injury in an mK(ATP)-dependent manner. Overall, these results suggest that the mK(ATP) channel is activated by specific reactive oxygen and nitrogen species, and inhibited by NADPH. The redox modulation of mK(ATP) may be an underlying mechanism for its regulation in the context of IPC. This article is part of a Special Issue entitled: Mitochondria and Cardioprotection.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures



Similar articles
-
A novel mitochondrial K(ATP) channel assay.Circ Res. 2010 Apr 16;106(7):1190-6. doi: 10.1161/CIRCRESAHA.109.215400. Epub 2010 Feb 25. Circ Res. 2010. PMID: 20185796 Free PMC article.
-
The complex II inhibitor atpenin A5 protects against cardiac ischemia-reperfusion injury via activation of mitochondrial KATP channels.Basic Res Cardiol. 2009 Mar;104(2):121-9. doi: 10.1007/s00395-009-0001-y. Epub 2009 Feb 26. Basic Res Cardiol. 2009. PMID: 19242645 Free PMC article.
-
Mitochondrial reactive oxygen species: which ROS signals cardioprotection?Am J Physiol Heart Circ Physiol. 2013 Oct 1;305(7):H960-8. doi: 10.1152/ajpheart.00858.2012. Epub 2013 Aug 2. Am J Physiol Heart Circ Physiol. 2013. PMID: 23913710 Free PMC article.
-
Mitochondrial K(ATP) channels: role in cardioprotection.Cardiovasc Res. 2002 Aug 15;55(3):429-37. doi: 10.1016/s0008-6363(02)00439-x. Cardiovasc Res. 2002. PMID: 12160940 Review.
-
The Slo(w) path to identifying the mitochondrial channels responsible for ischemic protection.Biochem J. 2017 Jun 9;474(12):2067-2094. doi: 10.1042/BCJ20160623. Biochem J. 2017. PMID: 28600454 Free PMC article. Review.
Cited by
-
Regulation of ion channels by pyridine nucleotides.Circ Res. 2013 Feb 15;112(4):721-41. doi: 10.1161/CIRCRESAHA.111.247940. Circ Res. 2013. PMID: 23410881 Free PMC article. Review.
-
Angiotensin receptor blockade recovers hepatic UCP2 expression and aconitase and SDH activities and ameliorates hepatic oxidative damage in insulin resistant rats.Endocrinology. 2012 Dec;153(12):5746-59. doi: 10.1210/en.2012-1390. Epub 2012 Oct 18. Endocrinology. 2012. PMID: 23087176 Free PMC article.
-
Angiotensin II-induced production of mitochondrial reactive oxygen species: potential mechanisms and relevance for cardiovascular disease.Antioxid Redox Signal. 2013 Oct 1;19(10):1085-94. doi: 10.1089/ars.2012.4604. Epub 2012 May 21. Antioxid Redox Signal. 2013. PMID: 22443458 Free PMC article. Review.
-
Role of mitochondrial oxidative stress in hypertension.Am J Physiol Heart Circ Physiol. 2013 Nov 15;305(10):H1417-27. doi: 10.1152/ajpheart.00089.2013. Epub 2013 Sep 16. Am J Physiol Heart Circ Physiol. 2013. PMID: 24043248 Free PMC article. Review.
-
Metabolic Stress.Arterioscler Thromb Vasc Biol. 2019 Jun;39(6):991-997. doi: 10.1161/ATVBAHA.118.312196. Arterioscler Thromb Vasc Biol. 2019. PMID: 31070466 Free PMC article. Review.
References
-
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. - PubMed
-
- Facundo HT, Fornazari M, Kowaltowski AJ. Tissue protection mediated by mitochondrial K+ channels. Biochim. Biophys. Acta. 2006;1762:202–212. - PubMed
-
- Garlid KD, Dos SP, Xie ZJ, Costa AD, Paucek P. Mitochondrial potassium transport: the role of the mitochondrial ATP-sensitive K(+) channel in cardiac function and cardioprotection. Biochim. Biophys. Acta. 2003;1606:1–21. - PubMed
-
- Murphy E, Steenbergen C. Preconditioning: the mitochondrial connection. Annu. Rev. Physiol. 2007;69:51–67. - PubMed
-
- Vanden Hoek TL, Becker LB, Shao Z, Li C, Schumacker PT. Reactive oxygen species released from mitochondria during brief hypoxia induce preconditioning in cardiomyocytes. J. Biol. Chem. 1998;273:18092–18098. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

