Genetic and epigenetic underpinnings of sex differences in the brain and in neurological and psychiatric disease susceptibility
- PMID: 21094887
- PMCID: PMC4465286
- DOI: 10.1016/B978-0-444-53630-3.00006-3
Genetic and epigenetic underpinnings of sex differences in the brain and in neurological and psychiatric disease susceptibility
Abstract
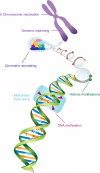
There are numerous examples of sex differences in brain and behavior and in susceptibility to a broad range of brain diseases. For example, gene expression is sexually dimorphic during brain development, adult life, and aging. These differences are orchestrated by the interplay between genetic, hormonal, and environmental influences. However, the molecular mechanisms that underpin these differences have not been fully elucidated. Because recent studies have highlighted the key roles played by epigenetic processes in regulating gene expression and mediating brain form and function, this chapter reviews emerging evidence that shows how epigenetic mechanisms including DNA methylation, histone modifications, and chromatin remodeling, and non-coding RNAs (ncRNAs) are responsible for promoting sexual dimorphism in the brain. Differential profiles of DNA methylation and histone modifications are found in dimorphic brain regions such as the hypothalamus as a result of sex hormone exposure during developmental critical periods. The elaboration of specific epigenetic marks is also linked with regulating sex hormone signaling pathways later in life. Furthermore, the expression and function of epigenetic factors such as the methyl-CpG-binding protein, MeCP2, and the histone-modifying enzymes, UTX and UTY, are sexually dimorphic in the brain. ncRNAs are also implicated in promoting sex differences. For example, X inactivation-specific transcript (XIST) is a long ncRNA that mediates X chromosome inactivation, a seminal developmental process that is particularly important in brain. These observations imply that understanding epigenetic mechanisms, which regulate dimorphic gene expression and function, is necessary for developing a more comprehensive view of sex differences in brain. These emerging findings also suggest that epigenetic mechanisms are, in part, responsible for the differential susceptibility between males and females that is characteristic of a spectrum of neurological and psychiatric disorders.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures

References
-
- Adegbola A, Gao H, Sommer S, Browning M. A novel mutation in JARID1C/SMCX in a patient with autism spectrum disorder (ASD). American Journal of Medical Genetics. Part A. 2008;146A:505–511. - PubMed
-
- Agger K, Cloos PA, Christensen J, Pasini D, Rose S, Rappsilber J, et al. UTX and JMJD3 are histone H3K27 demethylases involved in HOX gene regulation and development. Nature. 2007;449:731–734. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

