Transmissible gastroenteritis virus (TGEV)-based vectors with engineered murine tropism express the rotavirus VP7 protein and immunize mice against rotavirus
- PMID: 21094967
- PMCID: PMC7111951
- DOI: 10.1016/j.virol.2010.10.036
Transmissible gastroenteritis virus (TGEV)-based vectors with engineered murine tropism express the rotavirus VP7 protein and immunize mice against rotavirus
Abstract
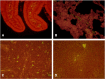
A coronavirus vector based on the genome of the porcine transmissible gastroenteritis virus (TGEV) expressing the rotavirus VP7 protein was constructed to immunize and protect against rotavirus infections in a murine model. The tropism of this TGEV-derived vector was modified by replacing the spike S protein with the homologous protein from mouse hepatitis virus (MHV). The rotavirus gene encoding the VP7 protein was cloned into the coronavirus cDNA. BALB/c and STAT1-deficient mice were inoculated with the recombinant viral vector rTGEV(S-MHV)-VP7, which replicates in the intestine and spreads to other organs such as liver, spleen and lungs. TGEV-specific antibodies were detected in all the inoculated BALB/c mice, while rotavirus-specific antibodies were found only after immunization by the intraperitoneal route. Partial protection against rotavirus-induced diarrhea was achieved in suckling BALB/c mice born to dams immunized with the recombinant virus expressing VP7 when they were orally challenged with the homotypic rotavirus strain.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Recombinant Lactobacillus plantarum NC8 strain expressing porcine rotavirus VP7 induces specific antibodies in BALB/c mice.Acta Biochim Biophys Sin (Shanghai). 2021 May 21;53(6):707-718. doi: 10.1093/abbs/gmab050. Acta Biochim Biophys Sin (Shanghai). 2021. PMID: 33963824
-
Homotypic protection against rotavirus-induced diarrhea in infant mice breast-fed by dams immunized with the recombinant VP8* subunit of the VP4 capsid protein.Viral Immunol. 2000;13(2):187-200. doi: 10.1089/vim.2000.13.187. Viral Immunol. 2000. PMID: 10892999
-
Immunogenicity and efficacy in mice of an adenovirus-based bicistronic rotavirus vaccine expressing NSP4 and VP7.Virus Res. 2015 Dec 2;210:298-307. doi: 10.1016/j.virusres.2015.09.010. Epub 2015 Sep 12. Virus Res. 2015. PMID: 26368053
-
Particle-bombardment-mediated DNA vaccination with rotavirus VP4 or VP7 induces high levels of serum rotavirus IgG but fails to protect mice against challenge.Virology. 1998 Oct 10;250(1):230-40. doi: 10.1006/viro.1998.9370. Virology. 1998. PMID: 9770437
-
Immunity to transmissible gastroenteritis virus and porcine respiratory coronavirus infections in swine.Vet Immunol Immunopathol. 1994 Oct;43(1-3):89-97. doi: 10.1016/0165-2427(94)90124-4. Vet Immunol Immunopathol. 1994. PMID: 7856068 Free PMC article. Review.
Cited by
-
Generation of a mouse scFv library specific for porcine aminopeptidase N using the T7 phage display system.J Virol Methods. 2012 Jun;182(1-2):99-103. doi: 10.1016/j.jviromet.2012.03.021. Epub 2012 Mar 28. J Virol Methods. 2012. PMID: 22481024 Free PMC article.
-
Antigenic structures stably expressed by recombinant TGEV-derived vectors.Virology. 2014 Sep;464-465:274-286. doi: 10.1016/j.virol.2014.07.027. Epub 2014 Aug 9. Virology. 2014. PMID: 25108114 Free PMC article.
-
Infectious Bronchitis Virus as a Vector for the Expression of Heterologous Genes.PLoS One. 2013 Jun 26;8(6):e67875. doi: 10.1371/journal.pone.0067875. Print 2013. PLoS One. 2013. PMID: 23840781 Free PMC article.
-
Development of a Genetically Engineered Bivalent Vaccine against Porcine Epidemic Diarrhea Virus and Porcine Rotavirus.Viruses. 2022 Aug 9;14(8):1746. doi: 10.3390/v14081746. Viruses. 2022. PMID: 36016368 Free PMC article.
-
Establishment of an infectious clone of the porcine transmissible gastroenteritis virus and a study on the location and function of accessory protein 3.Front Cell Infect Microbiol. 2025 Jun 11;15:1609022. doi: 10.3389/fcimb.2025.1609022. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40568696 Free PMC article.
References
-
- Almazan F., Dediego M.L., Galan C., Escors D., Alvarez E., Ortego J., Sola I., Zuniga S., Alonso S., Moreno J.L., Nogales A., Capiscol C., Enjuanes L. Construction of a severe acute respiratory syndrome coronavirus infectious cDNA clone and a replicon to study coronavirus RNA synthesis. J. Virol. 2006;80(21):10900–10906. - PMC - PubMed
-
- Arias C.F., Ballado T., Plebanski M. Synthesis of the outer-capsid glycoprotein of the simian rotavirus SA11 in Escherichia coli. Gene. 1986;47(2–3):211–219. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

