LT-IIc, a new member of the type II heat-labile enterotoxin family, exhibits potent immunomodulatory properties that are different from those induced by LT-IIa or LT-IIb
- PMID: 21095251
- PMCID: PMC3035162
- DOI: 10.1016/j.vaccine.2010.11.020
LT-IIc, a new member of the type II heat-labile enterotoxin family, exhibits potent immunomodulatory properties that are different from those induced by LT-IIa or LT-IIb
Abstract
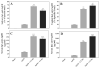
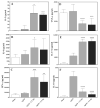
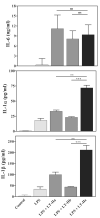
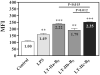
A plethora of human pathogens invade and/or colonize mucosal surfaces. Elaboration of strong, protective immune responses against those pathogens by mucosal vaccination, however, is hampered by endogenous regulatory systems in the mucosae that dampen responses to foreign antigens (Ags). To overcome those natural barriers, mucosal adjuvants must be employed. Using a mouse mucosal immunization model and AgI/II, a weak immunogen from Streptococcus mutans, LT-IIc, a new member of the type II subgroup of the heat-labile enterotoxin family, was shown to have potent mucosal adjuvant properties. In comparison to mice intranasally immunized only with AgI/II, co-administration of AgI/II with LT-IIc enhanced production of Ag-specific IgA antibodies in the saliva and vaginal fluids and Ag-specific IgA and IgG in the serum. Secretion of IL-2, IL-6, IL-17, IFN-γ, and TNF-α was enhanced in cultures of AgI/II-stimulated splenic cells isolated from mice that had received LT-IIc as a mucosal adjuvant. In contrast, secretion of IL-10 was suppressed in those cells. This pattern of cytokine secretion suggested that LT-IIc stimulates both Th1 and Th2 immune responses. In contrast to LT-IIa and LT-IIb, the original members of the type II subgroup that also are mucosal adjuvants, LT-IIc dramatically enhanced secretion of IL-1α and IL-1β in peritoneal macrophages that had been co-cultured with LPS. Furthermore, the B pentameric subunit of LT-IIc augmented uptake of Ag by bone marrow-derived dendritic cells to levels that exceeded those attained by use of LPS or by the B pentamers of LT-IIa or LT-IIb. These data confirmed that LT-IIc is a strong mucosal adjuvant with immunomodulatory properties that are distinguishable from those of LT-IIa and LT-IIb and which has immunomodulatory properties that may be exploitable in vaccine development.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
Intradermal administration of the Type II heat-labile enterotoxins LT-IIb and LT-IIc of enterotoxigenic Escherichia coli enhances humoral and CD8+ T cell immunity to a co-administered antigen.PLoS One. 2014 Dec 23;9(12):e113978. doi: 10.1371/journal.pone.0113978. eCollection 2014. PLoS One. 2014. PMID: 25536061 Free PMC article.
-
Comparative analysis of the mucosal adjuvanticity of the type II heat-labile enterotoxins LT-IIa and LT-IIb.Infect Immun. 2000 Jan;68(1):281-7. doi: 10.1128/IAI.68.1.281-287.2000. Infect Immun. 2000. PMID: 10603399 Free PMC article.
-
Mucosal adjuvant properties of mutant LT-IIa and LT-IIb enterotoxins that exhibit altered ganglioside-binding activities.Infect Immun. 2005 Mar;73(3):1330-42. doi: 10.1128/IAI.73.3.1330-1342.2005. Infect Immun. 2005. PMID: 15731030 Free PMC article.
-
Non-toxic derivatives of LT as potent adjuvants.Vaccine. 2011 Feb 11;29(8):1538-44. doi: 10.1016/j.vaccine.2010.11.091. Epub 2010 Dec 14. Vaccine. 2011. PMID: 21163247 Review.
-
The role of ADP-ribosylation and G(M1)-binding activity in the mucosal immunogenicity and adjuvanticity of the Escherichia coli heat-labile enterotoxin and Vibrio cholerae cholera toxin.Immunol Cell Biol. 1998 Jun;76(3):270-9. doi: 10.1046/j.1440-1711.1998.00745.x. Immunol Cell Biol. 1998. PMID: 9682971 Review.
Cited by
-
Intradermal administration of the Type II heat-labile enterotoxins LT-IIb and LT-IIc of enterotoxigenic Escherichia coli enhances humoral and CD8+ T cell immunity to a co-administered antigen.PLoS One. 2014 Dec 23;9(12):e113978. doi: 10.1371/journal.pone.0113978. eCollection 2014. PLoS One. 2014. PMID: 25536061 Free PMC article.
-
The Quest for an HIV-1 Vaccine Adjuvant: Bacterial Toxins as New Potential Platforms.J Clin Cell Immunol. 2014 Jun;5(3):225. doi: 10.4172/2155-9899.1000225. Epub 2014 Jul 17. J Clin Cell Immunol. 2014. PMID: 27375924 Free PMC article.
-
Type II heat-labile enterotoxins: structure, function, and immunomodulatory properties.Vet Immunol Immunopathol. 2013 Mar 15;152(1-2):68-77. doi: 10.1016/j.vetimm.2012.09.034. Epub 2012 Sep 26. Vet Immunol Immunopathol. 2013. PMID: 23137790 Free PMC article. Review.
-
Cell clustering and delay/arrest in T-cell division implicate a novel mechanism of immune modulation by E. coli heat-labile enterotoxin B-subunits.Cell Immunol. 2015 Jun;295(2):150-62. doi: 10.1016/j.cellimm.2015.02.014. Epub 2015 Mar 5. Cell Immunol. 2015. PMID: 25880107 Free PMC article.
-
Type II heat-labile enterotoxins from 50 diverse Escherichia coli isolates belong almost exclusively to the LT-IIc family and may be prophage encoded.PLoS One. 2012;7(1):e29898. doi: 10.1371/journal.pone.0029898. Epub 2012 Jan 5. PLoS One. 2012. PMID: 22242186 Free PMC article.
References
-
- Arce S, Nawar HF, Muehlinghaus G, Russell MW, Connell TD. In vitro induction of immunoglobulin A (IgA)- and IgM-secreting plasma blasts by cholera toxin depends on T-cell help and is mediated by CD154 up-regulation and inhibition of gamma interferon synthesis. Infect Immun. 2007;75:1413–1423. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

