RTEL1: an essential helicase for telomere maintenance and the regulation of homologous recombination
- PMID: 21097466
- PMCID: PMC3061057
- DOI: 10.1093/nar/gkq1045
RTEL1: an essential helicase for telomere maintenance and the regulation of homologous recombination
Abstract
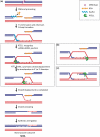
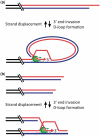
Telomere maintenance and DNA repair are crucial processes that protect the genome against instability. RTEL1, an essential iron-sulfur cluster-containing helicase, is a dominant factor that controls telomere length in mice and is required for telomere integrity. In addition, RTEL1 promotes synthesis-dependent strand annealing to direct DNA double-strand breaks into non-crossover outcomes during mitotic repair and in meiosis. Here, we review the role of RTEL1 in telomere maintenance and homologous recombination and discuss models linking RTEL1's enzymatic activity to its function in telomere maintenance and DNA repair.
Figures
References
-
- Blackburn EH. Switching and signaling at the telomere. Cell. 2001;106:661–673. - PubMed
-
- de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. - PubMed
-
- Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss H, de Lange T. Mammalian telomeres end in a large duplex loop. Cell. 1999;97:503–514. - PubMed

