Negative regulation of the endocytic adaptor disabled-2 (Dab2) in mitosis
- PMID: 21097498
- PMCID: PMC3037652
- DOI: 10.1074/jbc.M110.161851
Negative regulation of the endocytic adaptor disabled-2 (Dab2) in mitosis
Abstract
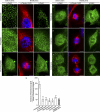
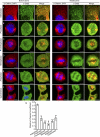
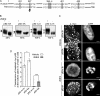
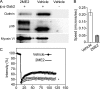
Mitotic cells undergo extensive changes in shape and size through the altered regulation and function of their membrane trafficking machinery. Disabled 2 (Dab2), a multidomain cargo-specific endocytic adaptor and a mediator of signal transduction, is a potential integrator of trafficking and signaling. Dab2 binds effectors of signaling and trafficking that localize to different intracellular compartments. Thus, differential localization is a putative regulatory mechanism of Dab2 function. Furthermore, Dab2 is phosphorylated in mitosis and is thus regulated in the cell cycle. However, a detailed description of the intracellular localization of Dab2 in the different phases of mitosis and an understanding of the functional consequences of its phosphorylation are lacking. Here, we show that Dab2 is progressively displaced from the membrane in mitosis. This phenomenon is paralleled by a loss of co-localization with clathrin. Both phenomena culminate in metaphase/anaphase and undergo partial recovery in cytokinesis. Treatment with 2-methoxyestradiol, which arrests cells at the spindle assembly checkpoint, induces the same effects observed in metaphase cells. Moreover, 2-methoxyestradiol also induced Dab2 phosphorylation and reduced Dab2/clathrin interactions, endocytic vesicle motility, clathrin exchange dynamics, and the internalization of a receptor endowed with an NPXY endocytic signal. Serine/threonine to alanine mutations, of residues localized to the central region of Dab2, attenuated its phosphorylation, reduced its membrane displacement, and maintained its endocytic abilities in mitosis. We propose that the negative regulation of Dab2 is part of an accommodation of the cell to the altered physicochemical conditions prevalent in mitosis, aimed at allowing endocytic activity throughout the cell cycle.
Figures

References
-
- Wang Z., Tseng C. P., Pong R. C., Chen H., McConnell J. D., Navone N., Hsieh J. T. (2002) J. Biol. Chem. 277, 12622–12631 - PubMed
-
- Huang C. H., Cheng J. C., Chen J. C., Tseng C. P. (2007) Cell. Signal. 19, 1339–1347 - PubMed
-
- Xu X. X., Yi T., Tang B., Lambeth J. D. (1998) Oncogene 16, 1561–1569 - PubMed
-
- Zhou J., Hsieh J. T. (2001) J. Biol. Chem. 276, 27793–27798 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

