Myristoylation of cGMP-dependent protein kinase dictates isoform specificity for serotonin transporter regulation
- PMID: 21097501
- PMCID: PMC3024740
- DOI: 10.1074/jbc.M110.203935
Myristoylation of cGMP-dependent protein kinase dictates isoform specificity for serotonin transporter regulation
Abstract
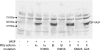
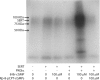
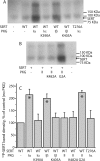
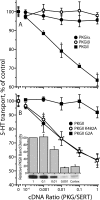
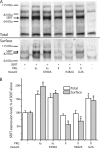
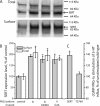
By transporting serotonin (5-HT) into neurons and other cells, serotonin transporter (SERT) modulates the action of 5-HT at cell surface receptors. SERT itself is modulated by several processes, including the cGMP signaling pathway. Activation of SERT by cGMP requires the cGMP-dependent protein kinase (PKG). Here we show that in HeLa cells lacking endogenous PKG, expression of PKGIα or PKGIβ was required for 8-bromoguanosine-3',5'-cyclic monophosphate (8-Br-cGMP) to stimulate SERT phosphorylation and 5-HT influx. Catalytically inactive PKG mutants and wild-type PKGII did not support this stimulation. However, a mutant PKGII (G2A) that was not myristoylated substituted for functional PKGI, suggesting that myristoylation and subsequent membrane association blocked productive interaction with SERT. PKG also influenced SERT expression and localization. PKGI isoforms increased total and cell surface SERT levels, and PKGII decreased cell surface SERT without altering total expression. Remarkably, these changes did not require 8-Br-cGMP or functional kinase activity and were also observed with a SERT mutant resistant to activation by PKG. Both PKGIα and PKGIβ formed detergent-stable complexes with SERT, and this association did not require catalytic activity. The nonmyristoylated PKGII G2A mutant stimulated SERT expression similar to PKGI isoforms. These results suggest multiple mechanisms by which PKG can modulate SERT and demonstrate that the functional difference between PKG isoforms results from myristoylation of PKGII.
Figures

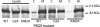

References
-
- Murphy D. L., Andrews A. M., Wichems C. H., Li Q., Tohda M., Greenberg B. (1998) J. Clin. Psychiatry 59, Suppl. 15, 4–12 - PubMed
-
- Stahl S. M. (1998) J. Affect. Disorders 51, 215–235 - PubMed
-
- Ramamoorthy S., Cool D. R., Mahesh V. B., Leibach F. H., Melikian H. E., Blakely R. D., Ganapathy V. (1993) J. Biol. Chem. 268, 21626–21631 - PubMed
-
- Jayanthi L. D., Ramamoorthy S., Mahesh V. B., Leibach F. H., Ganapathy V. (1994) J. Biol. Chem. 269, 14424–14429 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

