AlphaB-crystallin is found in detergent-resistant membrane microdomains and is secreted via exosomes from human retinal pigment epithelial cells
- PMID: 21097504
- PMCID: PMC3030331
- DOI: 10.1074/jbc.M110.160135
AlphaB-crystallin is found in detergent-resistant membrane microdomains and is secreted via exosomes from human retinal pigment epithelial cells
Abstract
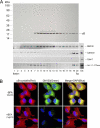
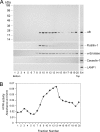
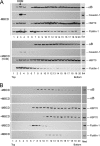
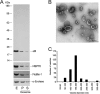
αB-crystallin (αB) is known as an intracellular Golgi membrane-associated small heat shock protein. Elevated levels of this protein have been linked with a myriad of neurodegenerative pathologies including Alzheimer disease, multiple sclerosis, and age-related macular degeneration. The membrane association of αB has been known for more than 3 decades, yet its physiological import has remained unexplained. In this investigation we show that αB is secreted from human adult retinal pigment epithelial cells via microvesicles (exosomes), independent of the endoplasmic reticulum-Golgi protein export pathway. The presence of αB in these lipoprotein structures was confirmed by its susceptibility to digestion by proteinase K only when exosomes were exposed to Triton X-100. Transmission electron microscopy was used to localize αB in immunogold-labeled intact and permeabilized microvesicles. The saucer-shaped exosomes, with a median diameter of 100-200 nm, were characterized by the presence of flotillin-1, α-enolase, and Hsp70, the same proteins that associate with detergent-resistant membrane microdomains (DRMs), which are known to be involved in their biogenesis. Notably, using polarized adult retinal pigment epithelial cells, we show that the secretion of αB is predominantly apical. Using OptiPrep gradients we demonstrate that αB resides in the DRM fraction. The secretion of αB is inhibited by the cholesterol-depleting drug, methyl β-cyclodextrin, suggesting that the physiological function of this protein and the regulation of its export through exosomes may reside in its association with DRMs/lipid rafts.
Figures





Similar articles
-
Alpha crystallins in the retinal pigment epithelium and implications for the pathogenesis and treatment of age-related macular degeneration.Biochim Biophys Acta. 2016 Jan;1860(1 Pt B):258-68. doi: 10.1016/j.bbagen.2015.05.016. Epub 2015 May 27. Biochim Biophys Acta. 2016. PMID: 26026469 Free PMC article. Review.
-
Inhibition of the Expression of the Small Heat Shock Protein αB-Crystallin Inhibits Exosome Secretion in Human Retinal Pigment Epithelial Cells in Culture.J Biol Chem. 2016 Jun 17;291(25):12930-42. doi: 10.1074/jbc.M115.698530. Epub 2016 Apr 27. J Biol Chem. 2016. PMID: 27129211 Free PMC article.
-
αB crystallin is apically secreted within exosomes by polarized human retinal pigment epithelium and provides neuroprotection to adjacent cells.PLoS One. 2010 Oct 8;5(10):e12578. doi: 10.1371/journal.pone.0012578. PLoS One. 2010. PMID: 20949024 Free PMC article.
-
Mutant Huntingtin Inhibits αB-Crystallin Expression and Impairs Exosome Secretion from Astrocytes.J Neurosci. 2017 Sep 27;37(39):9550-9563. doi: 10.1523/JNEUROSCI.1418-17.2017. Epub 2017 Sep 11. J Neurosci. 2017. PMID: 28893927 Free PMC article.
-
Novel roles for α-crystallins in retinal function and disease.Prog Retin Eye Res. 2012 Nov;31(6):576-604. doi: 10.1016/j.preteyeres.2012.06.001. Epub 2012 Jun 18. Prog Retin Eye Res. 2012. PMID: 22721717 Free PMC article. Review.
Cited by
-
Transcriptional Rearrangements Associated with Thermal Stress and Preadaptation in Baikal Whitefish (Coregonus baicalensis).Animals (Basel). 2024 Oct 25;14(21):3077. doi: 10.3390/ani14213077. Animals (Basel). 2024. PMID: 39518801 Free PMC article.
-
Role of exosomes in malignant glioma: microRNAs and proteins in pathogenesis and diagnosis.Cell Commun Signal. 2020 Aug 3;18(1):120. doi: 10.1186/s12964-020-00623-9. Cell Commun Signal. 2020. PMID: 32746854 Free PMC article. Review.
-
Propagation of Tau via Extracellular Vesicles.Front Neurosci. 2019 Jul 2;13:698. doi: 10.3389/fnins.2019.00698. eCollection 2019. Front Neurosci. 2019. PMID: 31312118 Free PMC article. Review.
-
Hsp20 functions as a novel cardiokine in promoting angiogenesis via activation of VEGFR2.PLoS One. 2012;7(3):e32765. doi: 10.1371/journal.pone.0032765. Epub 2012 Mar 12. PLoS One. 2012. PMID: 22427880 Free PMC article.
-
Alpha crystallins in the retinal pigment epithelium and implications for the pathogenesis and treatment of age-related macular degeneration.Biochim Biophys Acta. 2016 Jan;1860(1 Pt B):258-68. doi: 10.1016/j.bbagen.2015.05.016. Epub 2015 May 27. Biochim Biophys Acta. 2016. PMID: 26026469 Free PMC article. Review.
References
-
- Andley U. P. (2007) Prog. Retin. Eye Res. 26, 78–98 - PubMed
-
- Bhat S. P. (2003) Prog. Drug Res. 60, 205–262 - PubMed
-
- Horwitz J. (2000) Semin. Cell Dev. Biol. 11, 53–60 - PubMed
-
- Renkawek K., Voorter C. E., Bosman G. J., van Workum F. P., de Jong W. W. (1994) Acta Neuropathol. 87, 155–160 - PubMed
-
- D'Antonio M., Michalovich D., Paterson M., Droggiti A., Woodhoo A., Mirsky R., Jessen K. R. (2006) Glia 53, 501–515 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

