Tumor-secreted PGE2 inhibits CCL5 production in activated macrophages through cAMP/PKA signaling pathway
- PMID: 21097507
- PMCID: PMC3023508
- DOI: 10.1074/jbc.M110.154971
Tumor-secreted PGE2 inhibits CCL5 production in activated macrophages through cAMP/PKA signaling pathway
Abstract
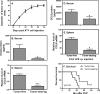
One of the major characteristics of tumors is their ability to evade immunosurveillance through altering the properties and functions of host stromal and/or immune cells. CCL5 has been shown to play important roles in T cell proliferation, IFN-γ, and IL-2 production, which promotes the differentiation and proliferation of Th1 cells important for immune defense against intracellular infection. In this study we found that tumor-bearing mice were more susceptible to bacterial infection and showed reduced CCL5 levels in serum during endotoxic shock. Our data further demonstrated that the soluble factors secreted by mammary gland tumor cells but not normal mammary gland epithelial cells inhibited CCL5 expression in macrophages in response to LPS, but not to TNF-α stimulation. The inhibitory effect of tumor-secreted molecules on LPS-induced CCL5 expression was regulated at the post-transcriptional level. Blocking PGE(2) synthesis by NS398 or through the use of PGE(2) receptor antagonists AH-6809 (EP2 antagonist) and AH-23848 (EP4 antagonist) completely reversed the inhibitory effect of tumor-conditioned medium (TCM) on LPS-induced CCL5 expression. Moreover, PGE(2) and the cAMP analog forskolin could mimic tumor-mediated CCL5 inhibition, and the inhibitory effects of TCM, PGE(2), and cAMP analog on LPS-induced CCL5 expression could be completely reversed by the PKA inhibitor H89. Furthermore, blocking PGE(2) synthesis in vivo led to partial recovery of CCL5 production during endotoxic shock. Taken together, our data indicate that PGE(2) secreted from breast cancer cells suppresses CCL5 secretion in LPS-activated macrophages through a cAMP/PKA signaling pathway, which may result in suppression of host immune responses against subsequent bacterial infection.
Figures



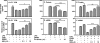

Similar articles
-
[Production and mechanism of CCL5 by macrophages in U14 cervical cancer-bearing mice during infection].Zhonghua Fu Chan Ke Za Zhi. 2015 May;50(5):367-73. Zhonghua Fu Chan Ke Za Zhi. 2015. PMID: 26311457 Chinese.
-
The ceramide-1-phosphate analogue PCERA-1 modulates tumour necrosis factor-alpha and interleukin-10 production in macrophages via the cAMP-PKA-CREB pathway in a GTP-dependent manner.Immunology. 2010 Mar;129(3):375-85. doi: 10.1111/j.1365-2567.2009.03188.x. Epub 2009 Nov 16. Immunology. 2010. PMID: 19922425 Free PMC article.
-
Effects of mechanical strain on the function of Gap junctions in osteocytes are mediated through the prostaglandin EP2 receptor.J Biol Chem. 2003 Oct 31;278(44):43146-56. doi: 10.1074/jbc.M302993200. Epub 2003 Aug 25. J Biol Chem. 2003. PMID: 12939279
-
Inducible cyclooxygenase released prostaglandin E2 modulates the severity of infection caused by Streptococcus pyogenes.J Immunol. 2010 Aug 15;185(4):2372-81. doi: 10.4049/jimmunol.1000838. Epub 2010 Jul 19. J Immunol. 2010. PMID: 20644176
-
Increased Th17 cells in the tumor microenvironment is mediated by IL-23 via tumor-secreted prostaglandin E2.J Immunol. 2013 Jun 1;190(11):5894-902. doi: 10.4049/jimmunol.1203141. Epub 2013 May 3. J Immunol. 2013. PMID: 23645882 Free PMC article.
Cited by
-
Identification of Caspase-6 as a New Regulator of Alternatively Activated Macrophages.J Biol Chem. 2016 Aug 12;291(33):17450-66. doi: 10.1074/jbc.M116.717868. Epub 2016 Jun 20. J Biol Chem. 2016. PMID: 27325699 Free PMC article.
-
Suppression of IL-12 production by tristetraprolin through blocking NF-kcyB nuclear translocation.J Immunol. 2013 Oct 1;191(7):3922-30. doi: 10.4049/jimmunol.1300126. Epub 2013 Aug 30. J Immunol. 2013. PMID: 23997224 Free PMC article.
-
Immunoregulatory mechanisms of the arachidonic acid pathway in cancer.FEBS Lett. 2025 Apr;599(7):927-951. doi: 10.1002/1873-3468.70013. Epub 2025 Feb 20. FEBS Lett. 2025. PMID: 39973474 Free PMC article. Review.
-
The Tumor-Promoting Flow of Cells Into, Within and Out of the Tumor Site: Regulation by the Inflammatory Axis of TNFα and Chemokines.Cancer Microenviron. 2012 Aug;5(2):151-64. doi: 10.1007/s12307-011-0094-3. Epub 2011 Dec 22. Cancer Microenviron. 2012. PMID: 22190050 Free PMC article.
-
E-type prostanoid receptor 4 (EP4) in disease and therapy.Pharmacol Ther. 2013 Jun;138(3):485-502. doi: 10.1016/j.pharmthera.2013.03.006. Epub 2013 Mar 21. Pharmacol Ther. 2013. PMID: 23523686 Free PMC article. Review.
References
-
- Nicolini A., Carpi A. (2009) Med. Res. Rev. 29, 436–471 - PubMed
-
- Baggiolini M. (1998) Nature 392, 565–568 - PubMed
-
- Luster A. D. (1998) N. Engl. J. Med. 338, 436–445 - PubMed
-
- Rollins B. J. (1997) Blood 90, 909–928 - PubMed
-
- Schall T. J., Jongstra J., Dyer B. J., Jorgensen J., Clayberger C., Davis M. M., Krensky A. M. (1988) J. Immunol. 141, 1018–1025 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

