Epigenomics: maternal high-fat diet exposure in utero disrupts peripheral circadian gene expression in nonhuman primates
- PMID: 21097519
- PMCID: PMC3228348
- DOI: 10.1096/fj.10-172080
Epigenomics: maternal high-fat diet exposure in utero disrupts peripheral circadian gene expression in nonhuman primates
Abstract
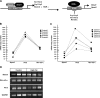
The effect of in utero exposure to a maternal high-fat diet on the peripheral circadian system of the fetus is unknown. Using mRNA copy number analysis, we report that the components of the peripheral circadian machinery are transcribed in the nonhuman primate fetal liver in an intact phase-antiphase fashion and that Npas2, a paralog of the Clock transcription factor, serves as the rate-limiting transcript by virtue of its relative low abundance (10- to 1000-fold lower). We show that exposure to a maternal high-fat diet in utero significantly alters the expression of fetal hepatic Npas2 (up to 7.1-fold, P<0.001) compared with that in control diet-exposed animals and is reversible in fetal offspring from obese dams reversed to a control diet (1.3-fold, P>0.05). Although the Npas2 promoter remains largely unmethylated, differential Npas2 promoter occupancy of acetylation of fetal histone H3 at lysine 14 (H3K14ac) occurs in response to maternal high-fat diet exposure compared with control diet-exposed animals. Furthermore, we find that disruption of Npas2 is consistent with high-fat diet exposure in juvenile animals, regardless of in utero diet exposure. In summary, the data suggest that peripheral Npas2 expression is uniquely vulnerable to diet exposure.
Figures


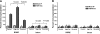
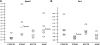
Similar articles
-
Developmental origins of disease and determinants of chromatin structure: maternal diet modifies the primate fetal epigenome.J Mol Endocrinol. 2008 Aug;41(2):91-102. doi: 10.1677/JME-08-0025. Epub 2008 May 30. J Mol Endocrinol. 2008. PMID: 18515302 Free PMC article.
-
Postweaning exposure to a high-fat diet is associated with alterations to the hepatic histone code in Japanese macaques.Pediatr Res. 2013 Sep;74(3):252-8. doi: 10.1038/pr.2013.106. Epub 2013 Jun 20. Pediatr Res. 2013. PMID: 23788059 Free PMC article.
-
High fat diet and in utero exposure to maternal obesity disrupts circadian rhythm and leads to metabolic programming of liver in rat offspring.PLoS One. 2014 Jan 9;9(1):e84209. doi: 10.1371/journal.pone.0084209. eCollection 2014. PLoS One. 2014. PMID: 24416203 Free PMC article.
-
Conditional postnatal deletion of the neonatal murine hepatic circadian gene, Npas2, alters the gut microbiome following restricted feeding.Am J Obstet Gynecol. 2017 Aug;217(2):218.e1-218.e15. doi: 10.1016/j.ajog.2017.03.024. Epub 2017 Mar 31. Am J Obstet Gynecol. 2017. PMID: 28373017 Free PMC article.
-
Dysregulation of Npas2 leads to altered metabolic pathways in a murine knockout model.Mol Genet Metab. 2013 Nov;110(3):378-87. doi: 10.1016/j.ymgme.2013.08.015. Epub 2013 Sep 5. Mol Genet Metab. 2013. PMID: 24067359 Free PMC article.
Cited by
-
Early origins of adult disease: approaches for investigating the programmable epigenome in humans, nonhuman primates, and rodents.ILAR J. 2012;53(3-4):306-21. doi: 10.1093/ilar.53.3-4.306. ILAR J. 2012. PMID: 23744969 Free PMC article.
-
Developmental Programming in Animal Models: Critical Evidence of Current Environmental Negative Changes.Reprod Sci. 2023 Feb;30(2):442-463. doi: 10.1007/s43032-022-00999-8. Epub 2022 Jun 13. Reprod Sci. 2023. PMID: 35697921 Free PMC article. Review.
-
The development and ecology of the Japanese macaque gut microbiome from weaning to early adolescence in association with diet.Am J Primatol. 2019 Oct;81(10-11):e22980. doi: 10.1002/ajp.22980. Epub 2019 May 7. Am J Primatol. 2019. PMID: 31066111 Free PMC article.
-
Interactions between Environmental Exposures and the Microbiome: Implications for Fetal Programming.Curr Opin Endocr Metab Res. 2020 Aug;13:39-48. doi: 10.1016/j.coemr.2020.09.003. Epub 2020 Oct 3. Curr Opin Endocr Metab Res. 2020. PMID: 33283070 Free PMC article.
-
Developmental Programming of the Fetal Immune System by Maternal Western-Style Diet: Mechanisms and Implications for Disease Pathways in the Offspring.Int J Mol Sci. 2024 May 29;25(11):5951. doi: 10.3390/ijms25115951. Int J Mol Sci. 2024. PMID: 38892139 Free PMC article. Review.
References
-
- Speiser P. W., Rudolf M. C., Anhalt H., Camacho-Hubner C., Chiarelli F., Eliakim A., Freemark M., Gruters A., Hershkovitz E., Iughetti L., Krude H., Latzer Y., Lustig R. H., Pescovitz O. H., Pinhas-Hamiel O., Rogol A. D., Shalitin S., Sultan C., Stein D., Vardi P., Werther G. A., Zadik Z., Zuckerman-Levin N., Hochberg Z. (2005) Childhood obesity. J. Clin. Endocrinol. Metab. 90, 1871–1887 - PubMed
-
- Grove K. L., Grayson B. E., Glavas M. M., Xiao X. Q., Smith M. S. (2005) Development of metabolic systems. Physiol. Behav. 86, 646–660 - PubMed
-
- Popkin B. M., Gordon-Larsen P. (2004) The nutrition transition: worldwide obesity dynamics and their determinants. Int. J. Obes. Relat. Metab. Disord. 28(Suppl. 3):S2–S9 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

