TNF/TNFR1 signaling mediates doxorubicin-induced diaphragm weakness
- PMID: 21097524
- PMCID: PMC3043812
- DOI: 10.1152/ajplung.00264.2010
TNF/TNFR1 signaling mediates doxorubicin-induced diaphragm weakness
Abstract
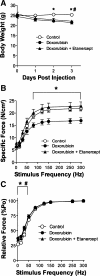
Doxorubicin, a common chemotherapeutic agent, causes respiratory muscle weakness in both patients and rodents. Tumor necrosis factor-α (TNF), a proinflammatory cytokine that depresses diaphragm force, is elevated following doxorubicin chemotherapy. TNF-induced diaphragm weakness is mediated through TNF type 1 receptor (TNFR1). These findings lead us to hypothesize that TNF/TNFR1 signaling mediates doxorubicin-induced diaphragm muscle weakness. We tested this hypothesis by treating C57BL/6 mice with a clinical dose of doxorubicin (20 mg/kg) via intravenous injection. Three days later, we measured contractile properties of muscle fiber bundles isolated from the diaphragm. We tested the involvement of TNF/TNFR1 signaling using pharmaceutical and genetic interventions. Etanercept, a soluble TNF receptor, and TNFR1 deficiency protected against the depression in diaphragm-specific force caused by doxorubicin. Doxorubicin stimulated an increase in TNFR1 mRNA and protein (P < 0.05) in the diaphragm, along with colocalization of TNFR1 to the plasma membrane. These results suggest that doxorubicin increases diaphragm sensitivity to TNF by upregulating TNFR1, thereby causing respiratory muscle weakness.
Figures




Similar articles
-
Doxorubicin causes diaphragm weakness in murine models of cancer chemotherapy.Muscle Nerve. 2011 Jan;43(1):94-102. doi: 10.1002/mus.21809. Muscle Nerve. 2011. PMID: 21171100 Free PMC article.
-
TNF-α/TNFR1 Signaling is Required for the Full Expression of Acute and Chronic Itch in Mice via Peripheral and Central Mechanisms.Neurosci Bull. 2018 Feb;34(1):42-53. doi: 10.1007/s12264-017-0124-3. Epub 2017 Apr 1. Neurosci Bull. 2018. PMID: 28365861 Free PMC article.
-
TNF-alpha acts via TNFR1 and muscle-derived oxidants to depress myofibrillar force in murine skeletal muscle.J Appl Physiol (1985). 2008 Mar;104(3):694-9. doi: 10.1152/japplphysiol.00898.2007. Epub 2008 Jan 10. J Appl Physiol (1985). 2008. PMID: 18187611
-
Beyond atrophy: redox mechanisms of muscle dysfunction in chronic inflammatory disease.J Physiol. 2011 May 1;589(Pt 9):2171-9. doi: 10.1113/jphysiol.2010.203356. Epub 2011 Feb 14. J Physiol. 2011. PMID: 21320886 Free PMC article. Review.
-
Chemotherapy-induced weakness and fatigue in skeletal muscle: the role of oxidative stress.Antioxid Redox Signal. 2011 Nov 1;15(9):2543-63. doi: 10.1089/ars.2011.3965. Epub 2011 Jun 15. Antioxid Redox Signal. 2011. PMID: 21457105 Free PMC article. Review.
Cited by
-
Liraglutide Pretreatment Does Not Improve Acute Doxorubicin-Induced Cardiotoxicity in Rats.Int J Mol Sci. 2024 May 27;25(11):5833. doi: 10.3390/ijms25115833. Int J Mol Sci. 2024. PMID: 38892020 Free PMC article.
-
Ameliorative effects of agomelatine against doxorubicin-induced hepatotoxicity.BMC Pharmacol Toxicol. 2025 Aug 8;26(1):146. doi: 10.1186/s40360-025-00988-y. BMC Pharmacol Toxicol. 2025. PMID: 40781695 Free PMC article.
-
The Impact of Immune Cells on the Skeletal Muscle Microenvironment During Cancer Cachexia.Front Physiol. 2020 Aug 31;11:1037. doi: 10.3389/fphys.2020.01037. eCollection 2020. Front Physiol. 2020. PMID: 32982782 Free PMC article. Review.
-
Doxorubicin causes cachexia, sarcopenia, and frailty characteristics in mice.PLoS One. 2024 Apr 22;19(4):e0301379. doi: 10.1371/journal.pone.0301379. eCollection 2024. PLoS One. 2024. PMID: 38648220 Free PMC article.
-
The Etiology and Impact of Muscle Wasting in Metastatic Cancer.Cold Spring Harb Perspect Med. 2020 Oct 1;10(10):a037416. doi: 10.1101/cshperspect.a037416. Cold Spring Harb Perspect Med. 2020. PMID: 31615873 Free PMC article. Review.
References
-
- Adams V, Mangner N, Gasch A, Krohne C, Gielen S, Hirner S, Thierse HJ, Witt CC, Linke A, Schuler G, Labeit S. Induction of MuRF1 is essential for TNF-alpha-induced loss of muscle function in mice. J Mol Biol 384: 48–59, 2008 - PubMed
-
- Arndt V, Stegmaier C, Ziegler H, Brenner H. A population-based study of the impact of specific symptoms on quality of life in women with breast cancer 1 year after diagnosis. Cancer 107: 2496–2503, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

