Requirements of the engineered leader peptide of nisin for inducing modification, export, and cleavage
- PMID: 21097596
- PMCID: PMC3020565
- DOI: 10.1128/AEM.01503-10
Requirements of the engineered leader peptide of nisin for inducing modification, export, and cleavage
Abstract
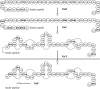
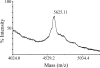
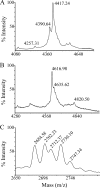
Nisin A is a pentacyclic peptide antibiotic produced by Lactococcus lactis. The leader peptide of prenisin keeps nisin inactive and has a role in inducing NisB- and NisC-catalyzed modifications of the propeptide and NisT-mediated export. The highly specific NisP cleaves off the leader peptide from fully modified and exported prenisin. We present here a detailed mutagenesis analysis of the nisin leader peptide. For alternative cleavage, we successfully introduced a putative NisP autocleavage site and sites for thrombin, enterokinase, Glu-C, and factor Xa in the C-terminal part of the leader peptide. Replacing residue F-18 with Trp or Thr strongly reduced production. On the other hand, D-19A, F-18H, F-18M, L-16D, L-16K, and L-16A enhanced production. Substitutions within and outside the FNLD box enhanced or reduced the transport efficiency. None of the above substitutions nor even an internal 6His tag from positions -13 to -8 had any effect on the capacity of the leader peptide to induce NisB and NisC modifications. Therefore, these data demonstrate a large mutational freedom. However, simultaneous replacement of the FNLD amino acids by four alanines strongly reduced export and even led to a complete loss of the capacity to induce modifications. Reducing the leader peptide to MSTKDFNLDLR led to 3- or 4-fold dehydration. Taken together, the FNLD box is crucial for inducing posttranslational modifications.
Figures


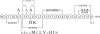
References
-
- Chatterjee, C., G. C. Patton, L. Cooper, M. Paul, and W. A. van der Donk. 2006. Engineering dehydro amino acids and thioethers into peptides using lacticin 481 synthetase. Chem. Biol. 13:1109-1117. - PubMed
-
- Chatterjee, C., M. Paul, L. Xie, and W. A. van der Donk. 2005. Biosynthesis and mode of action of lantibiotics. Chem. Rev. 105:633-684. - PubMed
-
- Chen, P., F. X. Qi, J. Novak, R. E. Krull, and P. W. Caufield. 2001. Effect of amino acid substitutions in conserved residues in the leader peptide on biosynthesis of the lantibiotic mutacin II. FEMS Microbiol. Lett. 195:139-144. - PubMed
-
- De Vries, L., et al. 2010. Oral and pulmonary delivery of thioether-bridged angiotensin-(1-7). Peptides 31:893-898. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

