Mycolic acid-containing bacteria induce natural-product biosynthesis in Streptomyces species
- PMID: 21097597
- PMCID: PMC3020563
- DOI: 10.1128/AEM.01337-10
Mycolic acid-containing bacteria induce natural-product biosynthesis in Streptomyces species
Abstract
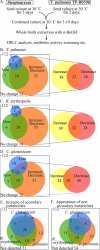
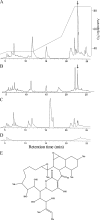
Natural products produced by microorganisms are important starting compounds for drug discovery. Secondary metabolites, including antibiotics, have been isolated from different Streptomyces species. The production of these metabolites depends on the culture conditions. Therefore, the development of a new culture method can facilitate the discovery of new natural products. Here, we show that mycolic acid-containing bacteria can influence the biosynthesis of cryptic natural products in Streptomyces species. The production of red pigment by Streptomyces lividans TK23 was induced by coculture with Tsukamurella pulmonis TP-B0596, which is a mycolic acid-containing bacterium. Only living cells induced this pigment production, which was not mediated by any substances. T. pulmonis could induce natural-product synthesis in other Streptomyces strains too: it altered natural-product biosynthesis in 88.4% of the Streptomyces strains isolated from soil. The other mycolic acid-containing bacteria, Rhodococcus erythropolis and Corynebacterium glutamicum, altered biosynthesis in 87.5 and 90.2% of the Streptomyces strains, respectively. The coculture broth of T. pulmonis and Streptomyces endus S-522 contained a novel antibiotic, which we named alchivemycin A. We concluded that the mycolic acid localized in the outer cell layer of the inducer bacterium influences secondary metabolism in Streptomyces, and this activity is a result of the direct interaction between the mycolic acid-containing bacteria and Streptomyces. We used these results to develop a new coculture method, called the combined-culture method, which facilitates the screening of natural products.
Figures


Similar articles
-
Killing of Mycolic Acid-Containing Bacteria Aborted Induction of Antibiotic Production by Streptomyces in Combined-Culture.PLoS One. 2015 Nov 6;10(11):e0142372. doi: 10.1371/journal.pone.0142372. eCollection 2015. PLoS One. 2015. PMID: 26544713 Free PMC article.
-
Intimate relationships among actinomycetes and mycolic acid-containing bacteria.Sci Rep. 2022 May 4;12(1):7222. doi: 10.1038/s41598-022-11406-2. Sci Rep. 2022. PMID: 35508597 Free PMC article.
-
AN483, a new anti-MRSA compound from Streptomyces sp.J Antibiot (Tokyo). 2016 Oct;69(10):762-764. doi: 10.1038/ja.2015.143. Epub 2016 Jan 13. J Antibiot (Tokyo). 2016. PMID: 26758490 No abstract available.
-
Taxonomy and biotransformation activities of some deep-sea actinomycetes.Extremophiles. 1998 Aug;2(3):269-77. doi: 10.1007/s007920050069. Extremophiles. 1998. PMID: 9783174 Review.
-
Novel antibiotic screening methods to awaken silent or cryptic secondary metabolic pathways in actinomycetes.J Antibiot (Tokyo). 2017 Jul;70(8):865-870. doi: 10.1038/ja.2017.51. Epub 2017 Apr 26. J Antibiot (Tokyo). 2017. PMID: 28442735 Review.
Cited by
-
Activation of silent biosynthetic pathways and discovery of novel secondary metabolites in actinomycetes by co-culture with mycolic acid-containing bacteria.J Ind Microbiol Biotechnol. 2019 Mar;46(3-4):363-374. doi: 10.1007/s10295-018-2100-y. Epub 2018 Nov 28. J Ind Microbiol Biotechnol. 2019. PMID: 30488365 Review.
-
Unique Physiological and Genetic Features of Ofloxacin-Resistant Streptomyces Mutants.Appl Environ Microbiol. 2022 Feb 8;88(3):e0232721. doi: 10.1128/aem.02327-21. Epub 2021 Dec 22. Appl Environ Microbiol. 2022. PMID: 34936843 Free PMC article.
-
Exploring Structural Diversity of Microbe Secondary Metabolites Using OSMAC Strategy: A Literature Review.Front Microbiol. 2019 Feb 26;10:294. doi: 10.3389/fmicb.2019.00294. eCollection 2019. Front Microbiol. 2019. PMID: 30863377 Free PMC article. Review.
-
Leveraging Experimental Strategies to Capture Different Dimensions of Microbial Interactions.Front Microbiol. 2021 Sep 27;12:700752. doi: 10.3389/fmicb.2021.700752. eCollection 2021. Front Microbiol. 2021. PMID: 34646243 Free PMC article. Review.
-
Let microorganisms do the talking, let us talk more about microorganisms.Fungal Biol Biotechnol. 2016 Jul 21;3:5. doi: 10.1186/s40694-016-0023-9. eCollection 2016. Fungal Biol Biotechnol. 2016. PMID: 28955464 Free PMC article.
References
-
- Bentley, S. D., et al. 2002. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141-147. - PubMed
-
- Cueto, M., et al. 2001. Pestalone, a new antibiotic produced by a marine fungus in response to bacterial challenge. J. Nat. Prod. 64:1444-1446. - PubMed
-
- Doull, J. L., and L. C. Vining. 1990. Nutritional control of actinorhodin production by Streptomyces coelicolor A3(2): suppressive effects of nitrogen and phosphate. Appl. Microbiol. Biotechnol. 32:449-454. - PubMed
-
- Feitelson, J. S., F. Malpartida, and D. A. Hopwood. 1985. Genetic and biochemical characterization of the red gene cluster of Streptomyces coelicolor A3(2). J. Gen. Microbiol. 131:2431-2441. - PubMed
-
- Flardh, K., and M. J. Buttner. 2009. Streptomyces morphogenetics: dissecting differentiation in a filamentous bacterium. Nat. Rev. Microbiol. 7:36-49. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

