Cannabinoid receptors, CB1 and CB2, as novel targets for inhibition of non-small cell lung cancer growth and metastasis
- PMID: 21097714
- PMCID: PMC3025486
- DOI: 10.1158/1940-6207.CAPR-10-0181
Cannabinoid receptors, CB1 and CB2, as novel targets for inhibition of non-small cell lung cancer growth and metastasis
Abstract
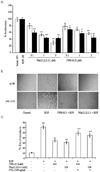
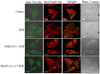
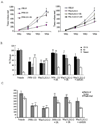
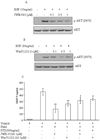
Non-small cell lung cancer (NSCLC) is the leading cause of cancer deaths worldwide; however, only limited therapeutic treatments are available. Hence, we investigated the role of cannabinoid receptors, CB1 and CB2, as novel therapeutic targets against NSCLC. We observed expression of CB1 (24%) and CB2 (55%) in NSCLC patients. Furthermore, we have shown that the treatment of NSCLC cell lines (A549 and SW-1573) with CB1/CB2- and CB2-specific agonists Win55,212-2 and JWH-015, respectively, significantly attenuated random as well as growth factor-directed in vitro chemotaxis and chemoinvasion in these cells. We also observed significant reduction in focal adhesion complex, which plays an important role in migration, upon treatment with both JWH-015 and Win55,212-2. In addition, pretreatment with CB1/CB2 selective antagonists, AM251 and AM630, prior to JWH-015 and Win55,212-2 treatments, attenuated the agonist-mediated inhibition of in vitro chemotaxis and chemoinvasion. In addition, both CB1 and CB2 agonists Win55,212-2 and JWH-133, respectively, significantly inhibited in vivo tumor growth and lung metastasis (∼50%). These effects were receptor mediated, as pretreatment with CB1/CB2 antagonists abrogated CB1/CB2 agonist-mediated effects on tumor growth and metastasis. Reduced proliferation and vascularization, along with increased apoptosis, were observed in tumors obtained from animals treated with JWH-133 and Win55,212-2. Upon further elucidation into the molecular mechanism, we observed that both CB1 and CB2 agonists inhibited phosphorylation of AKT, a key signaling molecule controlling cell survival, migration, and apoptosis, and reduced matrix metalloproteinase 9 expression and activity. These results suggest that CB1 and CB2 could be used as novel therapeutic targets against NSCLC.
©2010 AACR.
Conflict of interest statement
The authors declare that they have no potential conflicts of interest.
Figures


References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. - PubMed
-
- Kotsakis A, Georgoulias V. Targeting epidermal growth factor receptor in the treatment of non-small-cell lung cancer. Expert Opin Pharmacother - PubMed
-
- Franklin WA, Veve R, Hirsch FR, Helfrich BA, Bunn PA., Jr Epidermal growth factor receptor family in lung cancer and premalignancy. Semin Oncol. 2002;29:3–14. - PubMed
-
- Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183–232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

