Oxidative inactivation of the lipid phosphatase phosphatase and tensin homolog on chromosome ten (PTEN) as a novel mechanism of acquired long QT syndrome
- PMID: 21097842
- PMCID: PMC3024780
- DOI: 10.1074/jbc.M110.125526
Oxidative inactivation of the lipid phosphatase phosphatase and tensin homolog on chromosome ten (PTEN) as a novel mechanism of acquired long QT syndrome
Abstract
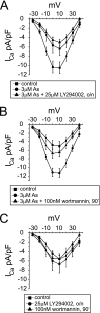
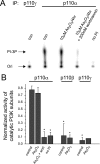
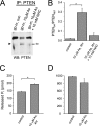
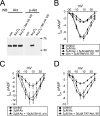
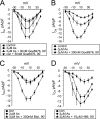
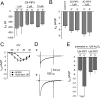
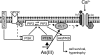
The most common cause of cardiac side effects of pharmaco-therapy is acquired long QT syndrome, which is characterized by abnormal cardiac repolarization and most often caused by direct blockade of the cardiac potassium channel human ether a-go-go-related gene (hERG). However, little is known about therapeutic compounds that target ion channels other than hERG. We have discovered that arsenic trioxide (As(2)O(3)), a very potent antineoplastic compound for the treatment of acute promyelocytic leukemia, is proarrhythmic via two separate mechanisms: a well characterized inhibition of hERG/I(Kr) trafficking and a poorly understood increase of cardiac calcium currents. We have analyzed the latter mechanism in the present study using biochemical and electrophysiological methods. We find that oxidative inactivation of the lipid phosphatase PTEN by As(2)O(3) enhances cardiac calcium currents in the therapeutic concentration range via a PI3Kα-dependent increase in phosphatidylinositol 3,4,5-triphosphate (PIP(3)) production. In guinea pig ventricular myocytes, even a modest reduction in PTEN activity is sufficient to increase cellular PIP(3) levels. Under control conditions, PIP(3) levels are kept low by PTEN and do not affect calcium current amplitudes. Based on pharmacological experiments and intracellular infusion of PIP(3), we propose that in guinea pig ventricular myocytes, PIP(3) regulates calcium currents independently of the protein kinase Akt along a pathway that includes a secondary oxidation-sensitive target. Overall, our report describes a novel form of acquired long QT syndrome where the target modified by As(2)O(3) is an intracellular signaling cascade.
Figures

Similar articles
-
Arsenic-induced interstitial myocardial fibrosis reveals a new insight into drug-induced long QT syndrome.Cardiovasc Res. 2012 Oct 1;96(1):90-8. doi: 10.1093/cvr/cvs230. Epub 2012 Aug 1. Cardiovasc Res. 2012. PMID: 22853924
-
Mechanisms of arsenic-induced prolongation of cardiac repolarization.Mol Pharmacol. 2004 Jul;66(1):33-44. doi: 10.1124/mol.66.1.33. Mol Pharmacol. 2004. PMID: 15213294
-
Arsenic trioxide-induced hERG K(+) channel deficiency can be rescued by matrine and oxymatrine through up-regulating transcription factor Sp1 expression.Biochem Pharmacol. 2013 Jan 1;85(1):59-68. doi: 10.1016/j.bcp.2012.09.002. Epub 2012 Oct 24. Biochem Pharmacol. 2013. PMID: 23103450
-
hERG channel trafficking: novel targets in drug-induced long QT syndrome.Biochem Soc Trans. 2007 Nov;35(Pt 5):1060-3. doi: 10.1042/BST0351060. Biochem Soc Trans. 2007. PMID: 17956279 Review.
-
The cardiac hERG/IKr potassium channel as pharmacological target: structure, function, regulation, and clinical applications.Curr Pharm Des. 2006;12(18):2271-83. doi: 10.2174/138161206777585102. Curr Pharm Des. 2006. PMID: 16787254 Review.
Cited by
-
Molecular determinants of pentamidine-induced hERG trafficking inhibition.Mol Pharmacol. 2012 Feb;81(2):198-209. doi: 10.1124/mol.111.075135. Epub 2011 Nov 1. Mol Pharmacol. 2012. PMID: 22046004 Free PMC article.
-
Incidence, Diagnosis, and Management of QT Prolongation Induced by Cancer Therapies: A Systematic Review.J Am Heart Assoc. 2017 Dec 7;6(12):e007724. doi: 10.1161/JAHA.117.007724. J Am Heart Assoc. 2017. PMID: 29217664 Free PMC article.
-
Redox signaling via oxidative inactivation of PTEN modulates pressure-dependent myogenic tone in rat middle cerebral arteries.PLoS One. 2013 Jul 5;8(7):e68498. doi: 10.1371/journal.pone.0068498. Print 2013. PLoS One. 2013. PMID: 23861911 Free PMC article.
-
Arsenite-induced stress signaling: modulation of the phosphoinositide 3'-kinase/Akt/FoxO signaling cascade.Redox Biol. 2013 Feb 4;1(1):104-9. doi: 10.1016/j.redox.2012.11.010. eCollection 2013. Redox Biol. 2013. PMID: 24024143 Free PMC article.
-
The rescuable function and mechanism of resveratrol on As₂O₃-induced hERG K⁺ channel deficiency.Naunyn Schmiedebergs Arch Pharmacol. 2014 Nov;387(11):1079-89. doi: 10.1007/s00210-014-1019-8. Epub 2014 Aug 10. Naunyn Schmiedebergs Arch Pharmacol. 2014. PMID: 25107562
References
-
- Nasr R., Guillemin M. C., Ferhi O., Soilihi H., Peres L., Berthier C., Rousselot P., Robledo-Sarmiento M., Lallemand-Breitenbach V., Gourmel B., Vitoux D., Pandolfi P. P., Rochette-Egly C., Zhu J., de Thé H. (2008) Nat. Med. 14, 1333–1342 - PubMed
-
- Wang Z. Y., Chen Z. (2008) Blood 111, 2505–2515 - PubMed
-
- Ohnishi K., Yoshida H., Shigeno K., Nakamura S., Fujisawa S., Naito K., Shinjo K., Fujita Y., Matsui H., Takeshita A., Sugiyama S., Satoh H., Terada H., Ohno R. (2000) Ann. Intern. Med. 133, 881–885 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

