The catalytic aspartate is protonated in the Michaelis complex formed between trypsin and an in vitro evolved substrate-like inhibitor: a refined mechanism of serine protease action
- PMID: 21097875
- PMCID: PMC3030363
- DOI: 10.1074/jbc.M110.161604
The catalytic aspartate is protonated in the Michaelis complex formed between trypsin and an in vitro evolved substrate-like inhibitor: a refined mechanism of serine protease action
Abstract
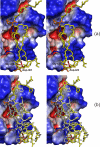
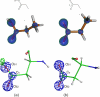
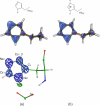
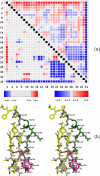
The mechanism of serine proteases prominently illustrates how charged amino acid residues and proton transfer events facilitate enzyme catalysis. Here we present an ultrahigh resolution (0.93 Å) x-ray structure of a complex formed between trypsin and a canonical inhibitor acting through a substrate-like mechanism. The electron density indicates the protonation state of all catalytic residues where the catalytic histidine is, as expected, in its neutral state prior to the acylation step by the catalytic serine. The carboxyl group of the catalytic aspartate displays an asymmetric electron density so that the O(δ2)-C(γ) bond appears to be a double bond, with O(δ2) involved in a hydrogen bond to His-57 and Ser-214. Only when Asp-102 is protonated on O(δ1) atom could a density functional theory simulation reproduce the observed electron density. The presence of a putative hydrogen atom is also confirmed by a residual mF(obs) - DF(calc) density above 2.5 σ next to O(δ1). As a possible functional role for the neutral aspartate in the active site, we propose that in the substrate-bound form, the neutral aspartate residue helps to keep the pK(a) of the histidine sufficiently low, in the active neutral form. When the histidine receives a proton during the catalytic cycle, the aspartate becomes simultaneously negatively charged, providing additional stabilization for the protonated histidine and indirectly to the tetrahedral intermediate. This novel proposal unifies the seemingly conflicting experimental observations, which were previously seen as either supporting the charge relay mechanism or the neutral pK(a) histidine theory.
Figures

Similar articles
-
Enzyme:substrate hydrogen bond shortening during the acylation phase of serine protease catalysis.Biochemistry. 2006 Feb 21;45(7):2114-21. doi: 10.1021/bi0517133. Biochemistry. 2006. PMID: 16475800
-
Role of Asp102 in the catalytic relay system of serine proteases: a theoretical study.J Am Chem Soc. 2004 Jun 9;126(22):7111-8. doi: 10.1021/ja030405u. J Am Chem Soc. 2004. PMID: 15174882
-
Energetically most likely substrate and active-site protonation sites and pathways in the catalytic mechanism of dihydrofolate reductase.J Am Chem Soc. 2001 Apr 18;123(15):3418-28. doi: 10.1021/ja0038474. J Am Chem Soc. 2001. PMID: 11472112
-
On the active site protonation state in aspartic proteases: implications for drug design.Curr Pharm Des. 2013;19(23):4257-75. doi: 10.2174/1381612811319230009. Curr Pharm Des. 2013. PMID: 23170891 Review.
-
Low-barrier hydrogen bonds and enzymatic catalysis.Arch Biochem Biophys. 2000 Oct 1;382(1):1-5. doi: 10.1006/abbi.2000.2011. Arch Biochem Biophys. 2000. PMID: 11051090 Review.
Cited by
-
Structural basis of bacterial defense against g-type lysozyme-based innate immunity.Cell Mol Life Sci. 2013 Mar;70(6):1113-22. doi: 10.1007/s00018-012-1184-1. Epub 2012 Oct 21. Cell Mol Life Sci. 2013. PMID: 23086131 Free PMC article.
-
Conformational dynamics of threonine 195 and the S1 subsite in functional trypsin variants.J Mol Model. 2012 Nov;18(11):4941-54. doi: 10.1007/s00894-012-1541-x. Epub 2012 Aug 8. J Mol Model. 2012. PMID: 22872415
-
Trypsinogen activation as observed in accelerated molecular dynamics simulations.Protein Sci. 2014 Nov;23(11):1550-8. doi: 10.1002/pro.2532. Epub 2014 Aug 23. Protein Sci. 2014. PMID: 25131668 Free PMC article.
-
Common Mechanism of Activated Catalysis in P-loop Fold Nucleoside Triphosphatases-United in Diversity.Biomolecules. 2022 Sep 22;12(10):1346. doi: 10.3390/biom12101346. Biomolecules. 2022. PMID: 36291556 Free PMC article.
-
Monospecific inhibitors show that both mannan-binding lectin-associated serine protease-1 (MASP-1) and -2 Are essential for lectin pathway activation and reveal structural plasticity of MASP-2.J Biol Chem. 2012 Jun 8;287(24):20290-300. doi: 10.1074/jbc.M112.354332. Epub 2012 Apr 16. J Biol Chem. 2012. PMID: 22511776 Free PMC article.
References
-
- Matthews B. W., Sigler P. B., Henderson R., Blow D. M. (1967) Nature 214, 652–656 - PubMed
-
- Blow D. M., Birktoft J. J., Hartley B. S. (1969) Nature 221, 337–340 - PubMed
-
- Markley J. L., Ibañez I. B. (1978) Biochemistry 17, 4627–4640 - PubMed
-
- Bachovchin W. W., Roberts J. D. (1978) J. Am. Chem. Soc. 100, 8041–8047
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

