Mechanisms of closed-state inactivation in voltage-gated ion channels
- PMID: 21098008
- PMCID: PMC3055536
- DOI: 10.1113/jphysiol.2010.191965
Mechanisms of closed-state inactivation in voltage-gated ion channels
Abstract
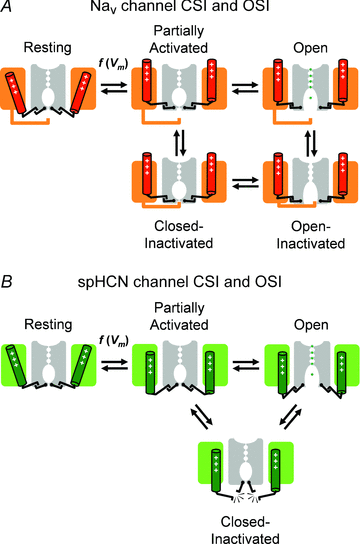
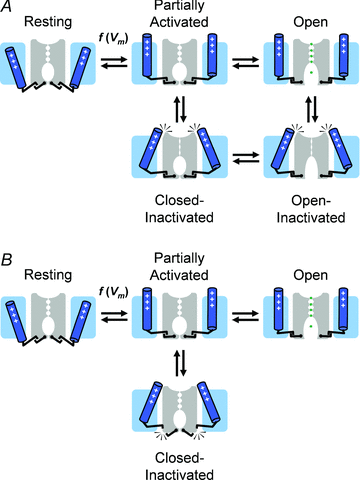
Inactivation of voltage-gated ion channels is an intrinsic auto-regulatory process necessary to govern the occurrence and shape of action potentials and establish firing patterns in excitable tissues. Inactivation may occur from the open state (open-state inactivation, OSI) at strongly depolarized membrane potentials, or from pre-open closed states (closed-state inactivation, CSI) at hyperpolarized and modestly depolarized membrane potentials. Voltage-gated Na(+), K(+), Ca(2+) and non-selective cationic channels utilize both OSI and CSI. Whereas there are detailed mechanistic descriptions of OSI, much less is known about the molecular basis of CSI. Here, we review evidence for CSI in voltage-gated cationic channels (VGCCs) and recent findings that shed light on the molecular mechanisms of CSI in voltage-gated K(+) (Kv) channels. Particularly, complementary observations suggest that the S4 voltage sensor, the S4S5 linker and the main S6 activation gate are instrumental in the installment of CSI in Kv4 channels. According to this hypothesis, the voltage sensor may adopt a distinct conformation to drive CSI and, depending on the stability of the interactions between the voltage sensor and the pore domain, a closed-inactivated state results from rearrangements in the selectivity filter or failure of the activation gate to open. Kv4 channel CSI may efficiently exploit the dynamics of the subthreshold membrane potential to regulate spiking properties in excitable tissues.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

