Membrane-impermeable cross-linking provides evidence for homophilic, isoform-specific binding of desmosomal cadherins in epithelial cells
- PMID: 21098030
- PMCID: PMC3023511
- DOI: 10.1074/jbc.M110.192245
Membrane-impermeable cross-linking provides evidence for homophilic, isoform-specific binding of desmosomal cadherins in epithelial cells
Abstract
Desmosomes and adherens junctions are cadherin-based protein complexes responsible for cell-cell adhesion of epithelial cells. Type 1 cadherins of adherens junctions show specific homophilic adhesion that plays a major role in developmental tissue segregation. The desmosomal cadherins, desmocollin and desmoglein, occur as several different isoforms with overlapping expression in some tissues where different isoforms are located in the same desmosomes. Although adhesive binding of desmosomal cadherins has been investigated in a variety of ways, their interaction in desmosome-forming epithelial cells has not been studied. Here, using extracellular homobifunctional cross-linking, we provide evidence for homophilic and isoform-specific binding between the Dsc2, Dsc3, Dsg2, and Dsg3 isoforms in HaCaT keratinocytes and show that it represents trans interaction. Furthermore, the cross-linked adducts are present in the detergent-insoluble fraction, and electron microscopy shows that extracellular cross-linking probably occurs in desmosomes. We found no evidence for either heterophilic or cis interaction, but neither can be completely excluded by our data. Mutation of amino acid residues Trp-2 and Ala-80 that are important for trans interaction in classical cadherin adhesive binding abolished Dsc2 binding, indicating that these residues are also involved in desmosomal adhesion. These interactions of desmosomal cadherins may be of key importance for their ordered arrangement within desmosomes that we believe is essential for desmosomal adhesive strength and the maintenance of tissue integrity.
Figures

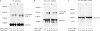
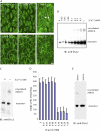




References
-
- Garrod D. R., Merritt A. J., Nie Z. (2002) Curr. Opin. Cell Biol. 14, 537–545 - PubMed
-
- Garrod D. R., Merritt A. J., Nie Z. (2002) Mol. Membr. Biol. 19, 81–94 - PubMed
-
- Kimura T. E., Merritt A. J., Garrod D. R. (2007) J. Invest. Dermatol. 127, 775–781 - PubMed
-
- Dusek R. L., Godsel L. M., Green K. J. (2007) J. Dermatol. Sci. 45, 7–21 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

